Access is everything for rare disease patients, who often face devastating conditions with no approved options.
Early access programmes (EAP) — also known as ‘expanded access programmes’ (US), ‘compassionate use programmes’ (EU) or ‘named-patient programmes’ (UK and parts of EU) — can play a critical role in bridging the gap between clinical studies and full rare drug commercialisation.
When strategically planned, EAPs can form the basis of a pharma market access strategy, generating insights, supporting regulatory pathways and even building payer familiarity ahead of launch.
This blog explains how small to mid-sized biotech and pharma companies — especially those working in rare disease indications — can effectively integrate EAPs into their broader access planning.
Why early access programmes should be part of rare drug commercialisation
Early access programmes allow patients with serious or life-threatening conditions to access investigational therapies before regulatory approval, usually when:
- No comparable or satisfactory alternatives exist.
- The patient is ineligible for ongoing clinical studies.
- The potential benefit justifies the risk.
- The company agrees to supply the product outside of commercial use.
There are some inherent challenges with rare drug commercialisation: small and widely dispersed patient populations, limited pre-existing real-world data, financial constraints and high therapy costs or manufacturing limits.
Although each market — be it the US, UK, EU or another territory — has unique legal, regulatory and logistical frameworks, the strategic value of early access programmes is global.
EAPs are often mistakenly viewed as purely medical or operational initiatives, when in reality they offer four clear strategic benefits aligned with commercial goals and your pharma market access strategy.
1. Generate real-world data (RWD) pre-launch
Many EAPs allow for the collection of observational, non-interventional data that can support:
- Real-world usage patterns.
- Safety signals.
- Health outcomes.
- Treatment burden insights.
This RWD is especially valuable in rare diseases, where traditional trial populations are small or lack diversity.
2. Build payer and health technology assessment (HTA) familiarity
EAPs allow physicians and institutions to experience the therapy firsthand. When structured properly, they help:
- Generate clinician testimonials or case studies.
- Prepare health systems operationally.
- Expose payers to real-world value prior to pricing negotiations.
3. Identify key treatment centres and key opinion leaders (KOLs)
Rare disease treatment is often centralised in a small number of specialised centres. EAPs can help identify and engage these hubs, which are often influential in shaping HTA outcomes and payer perceptions. So, focus on high-priority treatment centres and establish clear referral pathways.
4. Expand global reach in parallel to regulatory timelines
Global regulatory approvals don’t happen simultaneously. EAPs allow you to introduce your therapy earlier in high-need markets while awaiting formal launch.
Rather than opting for a blanket global rollout, use targeted programmes in these high-need markets and consider third-party support to help with implementation.
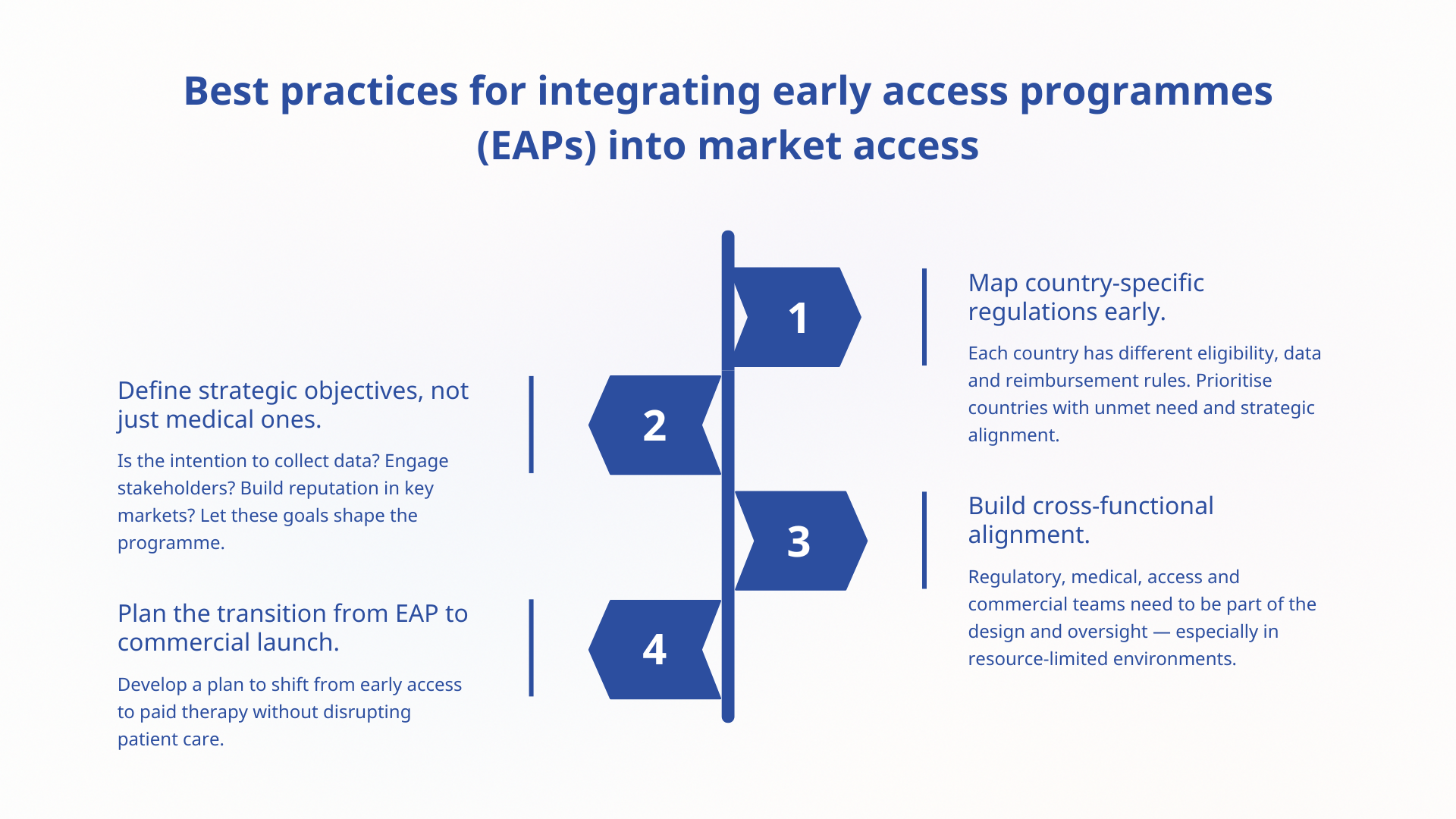
Key considerations for small biotech and pharma companies
Small companies may face limited resources, both in terms of infrastructure and personnel. Here are some key strategic and operational factors to help get it right:
Start planning early (pre-Phase III, if possible)
Early access programmes aren’t something you bolt on after clinical studies — they need to be built into an early access roadmap. Engagement is needed with:
- Drug regulatory affairs to navigate national requirements.
- Medical affairs to define eligibility criteria and education plans.
- Market access to identify where EAPs align with the pharma pricing strategy and reimbursement goals.
Consider which geographies are most strategically valuable to prioritise early access. Not all markets support EAPs or allow for data collection.
Balance compassion with operational feasibility
While the intent of EAPs is ethical, you should also consider:
- Supply chain logistics (especially for complex biologics or gene therapies).
- The cost of goods and pharma distribution.
- Internal bandwidth to handle requests and track outcomes
For rare diseases, treatment demand may be small but intense and urgent. Having clear processes (and possible third-party partners) is essential.
Align on your pharma pricing strategy
In some markets, EAPs can influence price expectations — especially if a drug is provided free of charge or through unstructured mechanisms.
To avoid unintended consequences:
- Track and document value delivered through EAPs.
- Prepare to justify the pharma pricing strategy with RWD and unmet need.
- Ensure early use doesn’t undermine price corridors in global reference pricing systems.
Leverage EAPs for stakeholder engagement
EAPs offer a unique touchpoint to:
- Build trust with physicians and treatment centres.
- Engage patient advocacy groups.
- Educate payers and HTA bodies on the burden of disease and treatment impact.
It’s advisable to use this opportunity to build champions and testimonials that support post-launch access and reimbursement.
By integrating early access programmes into your pharma market access strategy, you can serve patients faster, gather critical insights and establish credibility with payers and providers.
EAPs are not just a bridge between clinical studies and rare drug commercialisation — they’re a foundation for sustainable access and long-term impact.
TMC Commercial provides a full-service drug commercialisation solution, including regulatory compliance, marketing authorisation applications and pharma pricing strategy. If you want to reduce the time, cost and other complexities associated with entering new markets, contact TMC Commercial today at connect@tmcpharma.com.
