End-to-end solutions for all phases of drug development
From early-stage clinical development to market access and drug commercialisation, we provide a full range of integrated pharma services — covering all phases of drug development — for small to mid-sized biotech and pharma companies.
Our integrated pharma services simplify complex drug development pathways, transforming them into streamlined, patient-focused solutions that help you accelerate timelines while retaining control and maximising value.
Together, we can bring transformative therapies to patients faster.
Our integrated pharma services
Strategic support across your product lifecycle
From early-stage planning through clinical execution and commercial launch, our integrated pharma services provide strategic support and the right drug development solution at the right time in your product lifecycle.
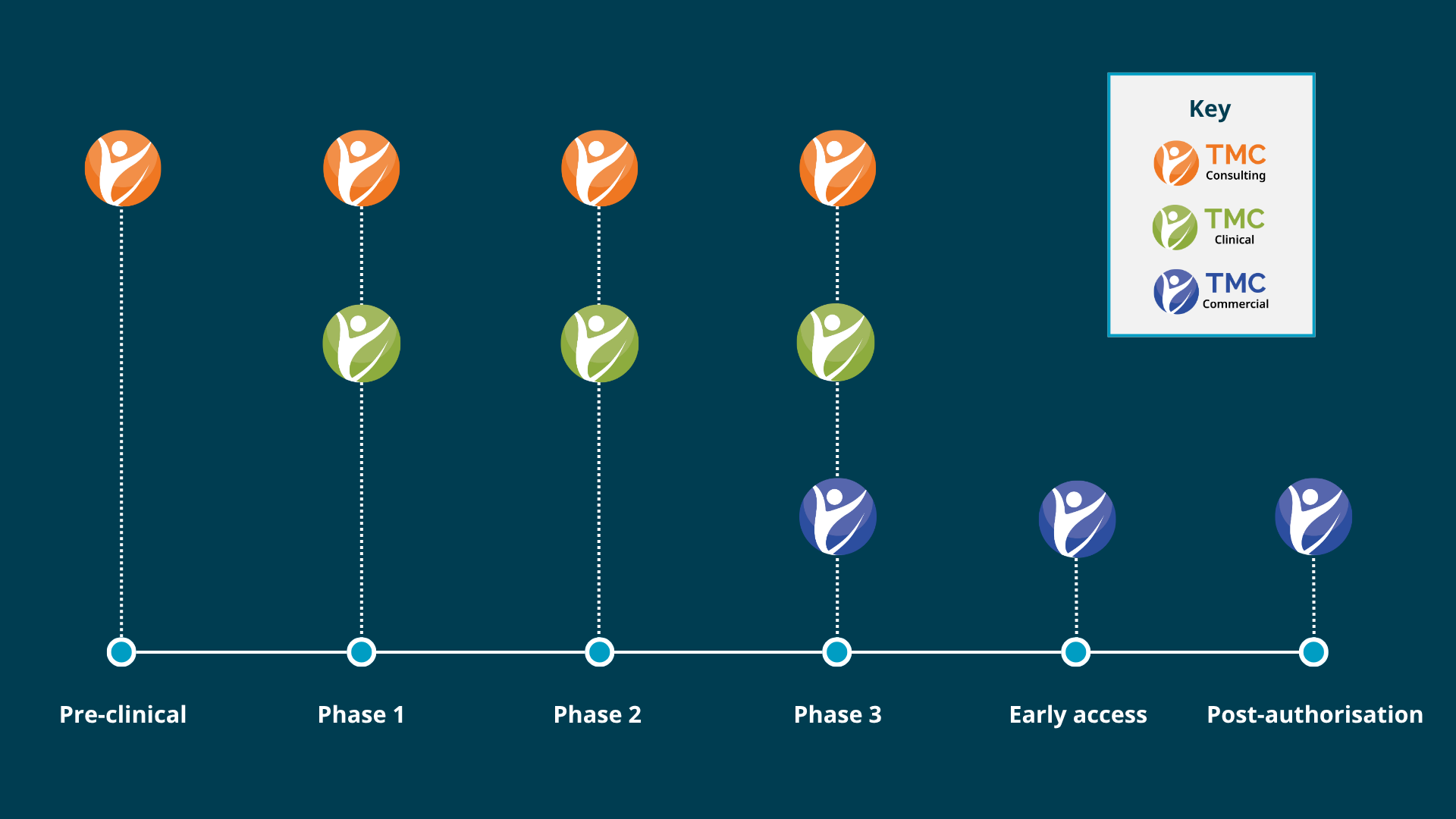
If you're looking to access cross-functional expertise to accelerate your drug development timeline, conduct seamless early-phase clinical trials for advanced therapeutics or gain EU and UK market access quickly and confidently, contact TMC today.
Our integrated pharma services and end-to-end support enable you to retain control, maximise value and bring transformative therapies to patients faster.



