Entering early‑phase clinical development with a novel advanced therapeutic modality brings tremendous opportunity — and significant complexity.
For small to mid‑sized biotech and pharma companies, designing a first‑in‑human or Phase 1/2 clinical trial requires a thoughtful strategy that’s tailored to the unique modality, efficient in resource use and aligned with regulatory expectations.
This blog outlines the essential considerations — and common challenges — to help you structure your early‑phase clinical trial with confidence.
Why novel advanced therapeutic modalities require special attention
Traditional early‑stage clinical trial study designs were developed around cytotoxic small molecules — assuming dose‑toxicity escalation relationships, relatively rapid pharmacokinetics in healthy volunteers or patients and well‑understood safety profiles.
For novel advanced therapeutic modalities, many of those assumptions may not hold. This is because:
- The mechanism of action may be longer term (e.g. gene‑editing, cell persistence), so safety/efficacy signals may emerge differently.
- Dose‑response or dose‑toxicity relationships may be non‑monotonic or difficult to predict (e.g. immune therapies).
- Biomarkers/pharmacodynamics may be critical to understanding the modality early (not just ‘what dose is tolerated?’).
- Regulatory authorities emphasise modality‑specific risks.
- Operationally and logistically, long‑term follow‑up and patient monitoring may all differ.
Because of these factors, early‑phase clinical trials for novel advanced therapeutics should be designed with extra care, combining scientific, statistical, operational and regulatory input from the outset.
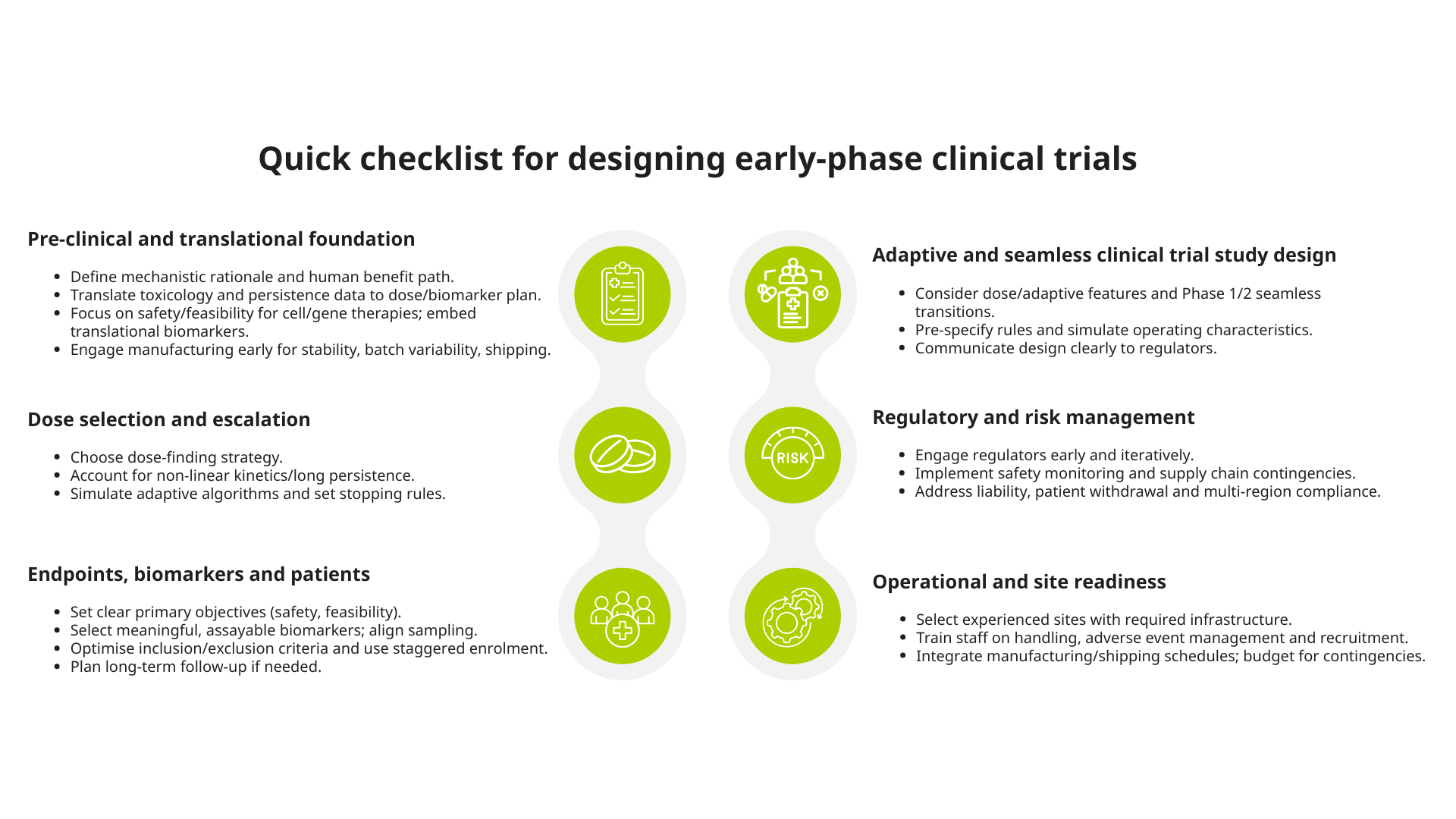
Six key considerations for designing early-phase clinical trials
These are some of the major areas that deserve careful attention when planning early-phase clinical trials (Phases 1/2/3) of novel advanced therapeutic modalities:
1. Pre-clinical and translational foundation
A robust translational foundation reduces scientific and regulatory risk, improves the likelihood that early human data is interpretable and prevents costly mid-stream changes — especially important if you can’t absorb large, unexpected drug development programme delays.
- Begin with a tightly articulated mechanistic rationale that links your modality to target biology and a plausible path to human benefit; translate non-clinical pharmacokinetics/ pharmacodynamics, toxicology and biodistribution/persistence data into a defensible human starting dose, escalation plan and biomarker strategy.
- For cell and gene therapies, frame early trials around safety, feasibility and tolerability rather than expecting definitive efficacy, and embed translational biomarkers (pharmacokinetics, pharmacodynamics, immunogenicity, cell persistence, etc.) to inform dose selection, patient stratification and early decisions.
- Engage manufacturing and chemistry, manufacturing and controls teams early — batch variability, stability, shipping and release testing for novel products frequently drive timelines and regulatory questions.
2. Dose selection and escalation strategy
The right escalation strategy protects patients, preserves trial integrity and finds the clinically relevant dose more efficiently — all of which conserve limited patient slots, budget and credibility for smaller developers.
- Move beyond reflexive ‘3+3’ designs when appropriate: decide whether your drug development programme should target a maximum tolerated dose, a minimum efficacious/biomarker-guided dose or some hybrid approach. Remember, many immuno- and cell-based modalities do not show monotonic toxicity/efficacy relationships.
- Account for non-linear kinetics or long persistence (which may require extended observation windows before escalating).
- Simulate any Bayesian or adaptive escalation algorithm to understand operating characteristics, and put clear stopping rules in place for toxicity, futility or biomarker non-response.
3. Endpoints, biomarkers and patient population
Well-chosen endpoints, biomarkers and populations produce high-value data that guide dose decisions and go/no-go choices, reduce false negatives/positives and satisfy regulators’ expectations — outcomes that speed development and preserve scarce resources.
- Define primary early-phase clinical development objectives clearly (usually safety, tolerability, pharmacokinetics/ pharmacodynamics, feasibility) and treat efficacy as secondary or exploratory unless justified otherwise.
- Select meaningful, assayable biomarkers for your modality (e.g. immune activation panels, cell infiltration, cytokines, transgene expression) and align sampling schedules to the modality’s kinetics. Choose inclusion/exclusion criteria and populations that maximise the chance of detecting a signal while protecting vulnerable participants.
- For high-risk or first-in-human clinical trials, use enrichment and staggered enrolment, and build long-term follow-up into the protocol where persistence or delayed effects are expected.
4. Adaptive and seamless clinical trial study design
Adaptive and seamless approaches can shorten timelines and make better use of patients and capital, but if poorly specified, they create interpretability, regulatory and operational risk — a trade-off you must manage carefully to avoid wasting the same limited resources you aim to conserve.
- Consider adaptive features (dose adaptation, response-adaptive randomisation, seamless Phase 1/2/3 transitions) to increase efficiency and reduce patient exposure, but recognise that adaptive trials demand pre-specified rules, comprehensive simulation of operating characteristics, early regulatory engagement and strong statistical and operational support.
- Seamless clinical trial study designs can accelerate development by combining dose finding with early efficacy cohorts, but they depend on correct assumptions and clear communication in protocol and with regulators to avoid ambiguity.
5. Regulatory and risk management
Proactive regulatory alignment and risk planning protect patient safety and reduce the chance of costly holds, protocol amendments or trial failures — all critical if you’re relying on milestone-driven financing or partnerships.
- Engage regulators (FDA, EMA, MHRA) early and iteratively; for novel advanced therapeutic modalities, regulators expect risk-based designs, appropriate monitoring and plans for long-term follow-up when relevant.
- Design a robust safety monitoring programme and assess manufacturing/supply chain contingencies — a single product quality or logistics failure can halt a trial.
- Address product liability, patient withdrawal plans and harmonise multi-region regulatory/ethics requirements and site capabilities if you plan global early-phase clinical development
6. Operational and site considerations
Execution matters as much as clinical trial study design — inexperienced sites, under-trained staff or optimistic timelines lead to data quality problems, enrolment delays and budget overruns, which are disproportionately damaging if you have limited reserves.
- Choose sites with the necessary experience and infrastructure (cellular therapy suites, specialised imaging, immunology labs).
- Invest in site training for product handling and adverse-event management.
- Design recruitment strategies that consider patient advocacy groups, eligibility burden and patient logistics.
- For cell and gene therapies, integrate manufacturing, shipping and treatment timing into realistic schedules, and budget for higher costs and timeline uncertainty with contingency plans.
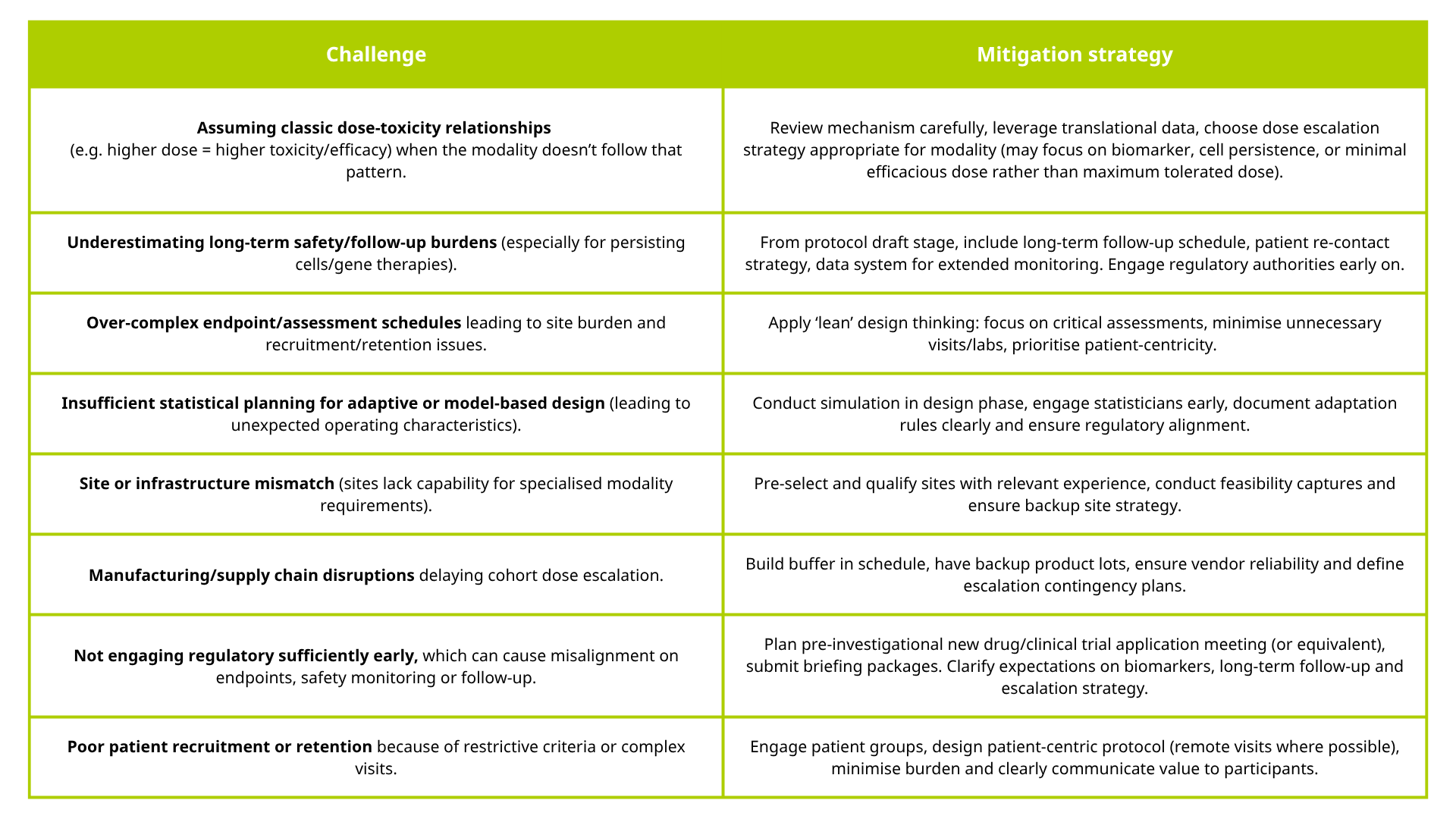
Common early-phase clinical development challenges and how to avoid them
Below are some of the stumbling blocks smaller biotech and pharma companies often come across during early-phase clinical trials of novel modalities — along with recommended mitigation strategies.
TMC Clinical provides early-phase clinical development solutions for rare disease, complex oncology and advanced therapeutic modalities. We combine the responsiveness of a specialist pharma services company with the expertise of a clinical research organisation, enabling you to accelerate your drug development timelines, reduce risk and move confidently towards global regulatory approvals.
Contact our team today at connect@tmcpharma.com to see how we can help you design and conduct seamless early-phase clinical trials for novel modalities.
