For small to mid‑sized biotech and pharma companies, building every function in‑house often means heavy headcount, fixed overheads and less agility. That’s where the functional service provider operating model can be highly beneficial.
The key is selecting the right functional service provider model to align with your business strategy, pipeline demands and internal capabilities.
This blog explains what a functional service provider (FSP) is and explores the two different operating models you could adopt, how to evaluate them and practical tips for successful deployment.
What is a functional service provider (FSP)?
A functional service provider is an outsourcing partner engaged to deliver one or more functions — rather than an entire programme — within your standard operating procedures (SOPs).
Compared with a full-service contract research organisation (CRO) model, where the CRO runs the entire trial or programme, the FSP model provides modularity, flexible resourcing and control.
Typical pharma services functions include:
- Clinical monitoring/operations.
- Data management and biostatistics.
- Medical writing.
- Drug regulatory affairs.
- Pharmacovigilance and safety monitoring.
- Quality management.
However, the traditional functional service provider model isn’t necessarily the best approach for small to mid-sized biotech and pharma companies that often face shifting pipelines and variable workloads.
Understanding the different functional service provider operating models
As your drug development pipeline evolves and resourcing needs fluctuate, one size rarely fits all when it comes to FSP models. For small to mid-sized biotech and pharma companies, selecting the right approach can make a big difference in flexibility, cost efficiency and operational control.
Below we outline two common models and how they differ — and when each might make sense.
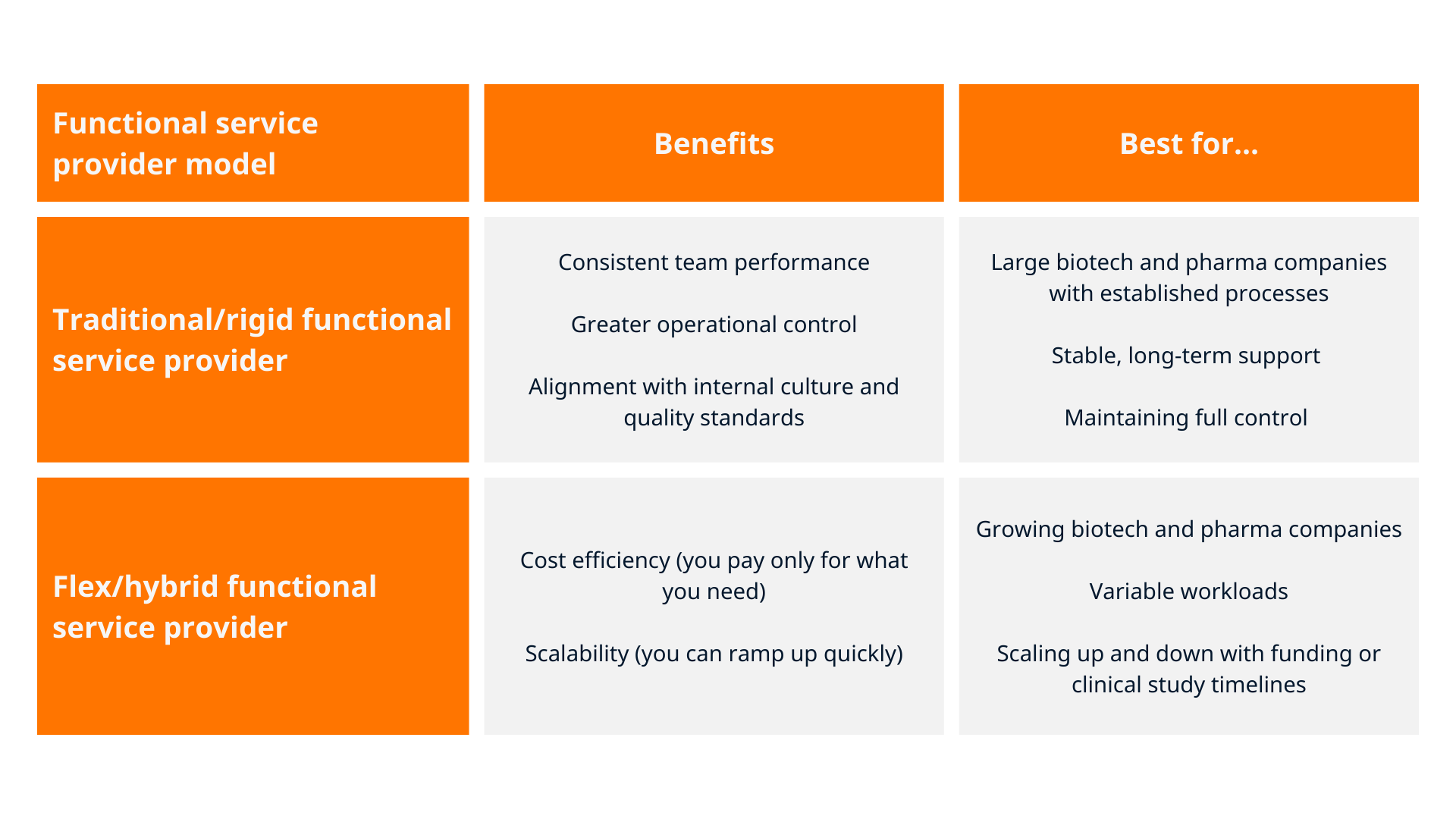
Traditional/rigid functional service provider
In this model, your functional service provider partner provides a dedicated team of full-time employees who work as an extension of your internal function to bring key pharma services into your project. They follow your SOPs, systems and processes, with clear governance and KPIs.
- FSP partner responsibilities: recruiting, onboarding, line management and training.
- Your responsibilities: strategy, SOP ownership and data governance.
- Benefits: consistent team performance, greater operational control and alignment with your internal culture and quality standards.
- Best for: larger organisations with established processes looking for stable, long-term support without outsourcing control.
Flex/hybrid functional service provider
This model builds on the traditional functional service provider model by adding a flexible ‘on-demand’ resource pool. A core team supports your ongoing needs, while additional resources can be added rapidly for peaks — for example, integrating a compliance expert during regulatory submissions or a clinical expert during major clinical study milestones.
- Benefits: cost efficiency (you pay only for what you need) and scalability (you can ramp up quickly).
- Best for: growing biotech and pharma companies with variable workloads or drug development programmes that scale up and down with funding or clinical study timelines.
The key is to match the model to function characteristics, your pipeline stage and cost-risk profile.
Why scaling biotech and pharma companies should consider a flex/hybrid functional service provider model
Whereas a traditional/rigid FSP model can lock you into mismatched resources and costs, a flex/hybrid FSP model lets you scale specialised expertise up or down quickly, align teams to evolving priorities and optimise both quality and spend.
Some of the main benefits associated with this functional service provider approach, especially for those companies in growth mode, include:
- Scalability and flexibility: the flex/hybrid FSP model enables you to scale up (or down) dedicated resources for a function in response to study peaks, submission deadlines or new asset launches.
- Cost efficiency/converting fixed cost to variable cost: rather than hiring permanent staff for a future programme, you can outsource the function and pay only for what’s needed.
- Access to highly specialist expertise: a flex/hybrid FSP partner brings subject‑matter experts with experience across sponsors and therapeutic areas, which is particularly helpful for emerging companies without deep internal resources.
- Retention of strategic control and transparency: the right partner will be able to integrate flexibly into your systems and SOPs, meaning you preserve oversight of data, quality and strategic direction.
- Operational continuity: minimising the burden of staff hiring, training and turnover can help maintain momentum in complex programmes.
For small to mid-sized biotech and pharma companies looking to grow, move assets through the pipeline and manage operational risk, the flex/hybrid functional service provider operating model is a powerful advantage.
A step-by-step guide on choosing the right functional service provider model for you
Step 1: clarify your strategic drivers and internal capabilities
Ask yourself:
- What are the business objectives for the coming 12–24 months (e.g. proof of concept, pivotal trial, regulatory submission, launch)?
- Which functions are mission‑critical vs support functions?
- Do we have internal infrastructure (SOPs, systems, qualified staff) for those functions?
- What is our resource demand profile (steady vs variable)?
These insights will guide whether you need a full-time dedicated team (traditional FSP) or a flexible model.
Step 2: map function characteristics
Evaluate the function(s) you’re considering outsourcing along parameters like:
- Volume and predictability of work (steady vs peaks).
- Complexity and required expertise (highly specialised).
- Regulatory/quality-risk (e.g. pharmacovigilance vs simple data entry).
- Integration needs (need access to sponsor systems, your SOPs).
For example, a data management function supporting multiple clinical studies may be steady and high volume, making it a good fit for a traditional functional service provider operating model. However, a function supporting a one‑time submission spike might suit a hybrid FSP model or even a short‑term partner.
Step 3: assess vendor fit and operating model flexibility
Key selection criteria:
- Does the FSP have a track record of embedding teams into sponsor systems/SOPs?
- Are contracts structured for scalability (flex up/down) and transparent cost models (FTE, deliverable‑based, hybrid)?
- How will governance, KPIs and reporting work? Lack of oversight is a risk.
- Does the FSP offer cultural fit, global delivery as required?
- What are the escalation/resourcing risks (e.g. vendor losing key staff, turnover)?
Choosing the wrong partner or model can negate the benefits of the functional service provider operating model.
Step 4: establish governance, metrics and integration
Operational success hinges on governance and integration. Recommended actions include:
- Defining clear roles and responsibilities between you and your FSP partner.
- Establishing KPIs (quality, timeline, cost, resource utilisation).
- Ensuring your FSP partner resources work in your systems or are aligned closely to your SOPs/data flows.
- Agreeing on escalation procedures for issues (resourcing, compliance, data integrity).
- Conducting onboarding/training of the FSP team to your systems and culture.
- Including contract flexibility (scale up, scale down, exit) aligned to your pipeline dynamics.
Step 5: review and optimise as your pipeline evolves
As your company scales, your needs will evolve — what made sense at the early stage of your drug development programme may change at a later phase. You should:
- Monitor utilisation and cost: are you under- or over‑resourced?
- Review the strategic fit: does the FSP model still align with internalisation plans (e.g. building in‑house capability)?
- Consider transition plans: at what point does bringing the function in‑house make sense vs continuing with your FSP?
- Leverage continuous improvement: use your FSP’s insights to optimise workflows, data flows and resource models.
Six common functional service provider pitfalls and how to avoid them
While the functional service provider operating model offers advantages, you should also be aware of these common pitfalls:
- Poor alignment of scope and expectations: if you haven’t defined the function clearly (volume, deliverables, role), the FSP may under‑deliver or cost more.
- Over‑ or under‑resourcing: not aligning resource levels to actual demand leads to wasted cost or missed milestones.
- Lack of integration: if the FSP works in isolation, data transfer issues, process misalignment and duplication of effort can result.
- Turnover/resourcing risks: if the FSP’s team changes frequently, continuity suffers.
- Governance gaps: without KPIs, oversight structure and escalation paths, you may lose control.
- Rigid contract models: if the contract doesn’t allow scaling flexibility, the benefits of the FSP model may be lost.
By aligning your internal capabilities, pipeline demands and growth strategy with a functional service provider model that fits, you can unlock scalability, cost efficiency and operational excellence while still retaining oversight and control.
TMC Consulting provides expert-led cross-functional integration across all stages of your product lifecycle. Our experienced specialists — many of whom have worked with us for years — act as an extension of your team, partnering flexibly with you to integrate key roles, such as a medic or regulatory expert, and pharma services into your project as required. Rather than taking over the entire drug development programme, we plug into the parts that need scale or specialist skills while you retain oversight and control.
Contact our team today at connect@tmcpharma.com to learn more about our pharma consulting services and see how we can help you scale operations efficiently and cost-effectively.
