The line between medical affairs and drug commercialisation functions can sometimes seem blurred — particularly for small to mid-sized biotech and pharma companies.
Resources are limited, teams are lean, and cross-functional collaboration is essential.
To maintain the integrity of science and compliance with regulations, it’s important to understand and respect the line between medical and commercial. This isn’t just about internal governance — it’s about building trust with stakeholders and protecting your asset.
This blog helps you navigate the complex, but essential, separation of these roles.
Why this dividing line matters beyond compliance
At its core, the divide between medical affairs and drug commercialisation is a philosophical one.
Medical teams serve the scientific, educational and clinical engagement needs of external stakeholders. Its role is non-promotional, focusing on truth, data integrity and patient outcomes.
Commercial teams drive the adoption and uptake of products through promotional activities, primarily directed at healthcare providers (HCPs), payers and systems.
Blurring these lines risks:
- Regulatory violations (e.g. off-label promotion).
- Damaged credibility with key opinion leaders (KOLs).
- Loss of trust from patient advocacy groups.
- Reputational and financial damage.
Emerging biotech and pharma companies frequently face two additional pressures that can inadvertently create confusion or tension between medical and commercial functions:
- Small teams, overlapping roles. Often, the same leaders wear multiple hats in early-stage companies. A Chief Medical Officer (CMO) might be overseeing both clinical development and medical services. A VP of Commercial may be pushing for early engagement in territories where the product isn’t even approved yet. As a result, medical voices may be co-opted for commercial purposes, leading to perceived bias or misalignment with scientific credibility.
- High-stakes, single-asset companies. For many emerging biotech and pharma companies, the company’s future depends on a single product. The pressure to demonstrate value and accelerate uptake post-approval is enormous. Commercial pressures may, therefore, push teams to engage prematurely — or inappropriately — with HCPs or payers, especially in global markets with varying regulatory standards.
Practical guidelines to drawing the line — clearly
Here are four tangible ways you can clarify and uphold the separation between your medical affairs and drug commercialisation teams:
1. Define roles and responsibilities early
Document clear responsibilities for medical and commercial teams — even if there is only one person per function:
Medical affairs:
- Scientific exchange with KOLs and HCPs (non-promotional)
- Medical education programmes
- Insight gathering for research and development and market access teams
- Real-world evidence generation
- Responding to unsolicited medical inquiries
Drug commercialisation:
- Product promotion (post-approval)
- Marketing and branding
- Sales training and materials
- Payer contracting and pharma pricing strategy (in coordination with market access)
2. Separate external engagement activities
Create firm boundaries around how and when each team engages externally. For example:
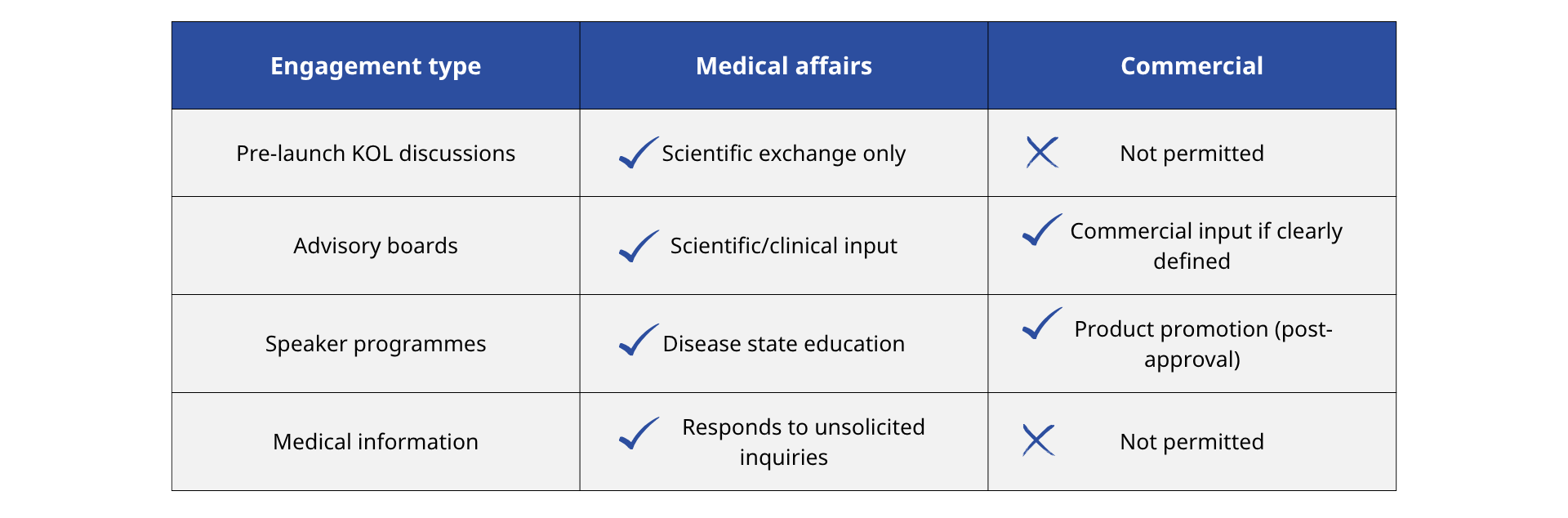
3. Train early and often on compliance
Invest in compliance training for everyone — not just legal or regulatory staff. Ensure medical and commercial leaders understand:
- FDA/EMA promotional rules.
- Off-label communication risks.
- Fair balance and substantiation requirements.
Also, don’t assume that new hires from larger companies will automatically follow the rules — startups often have different risk profiles.
4. Use governance processes
When collaboration is needed (e.g. advisory boards, launch planning), set up cross-functional governance with the right controls:
- Joint planning meetings with clear agendas and minutes.
- Pre-approved slides and materials.
- Independent roles in field engagement, e.g. medical science liaison (MSL) vs. sales rep boundaries.
- Medical sign-off on any scientific content used commercially.
Building a culture of integrity
KOLs, patients and regulators pay close attention to how data is presented, how you engage with the community and whether your actions reflect a commitment to science or sales.
By clearly delineating the role of medical affairs, you can stay compliant and establish a brand that’s rooted in scientific integrity, which is crucial in gaining trust, support and market access.
In short, you should:
- Define medical and commercial charters clearly.
- Ensure scientific exchanges are non-promotional.
- Establish compliance training from Day 1.
- Use cross-functional collaboration with controls.
- Invest in medical services early.
You shouldn’t:
- Let team members operate in undefined grey areas.
- Use MSLs as shadow sales reps.
- Wait until launch to focus on governance.
- Let commercial drive all external strategies.
- Treat medical as an afterthought post-approval.
Speed and agility are important for small to mid-sized biotech and pharma companies — but so is precision. Establishing a clear line between science and sales not only retains compliance, but it also helps you strengthen your brand, build trust with the community and set the foundation for long-term commercial success.
TMC Commercial provides a full-service drug commercialisation solution, including medical affairs, regulatory compliance and marketing authorisation application support. If you want to reduce the time, cost and other complexities associated with entering new markets, contact TMC Commercial today at connect@tmcpharma.com.
