Small and mid-sized biotechs and pharma companies live and breathe innovation — but when it comes to running clinical studies, limited budgets, lean teams and evolving regulatory expectations can slow even the best science.
That’s where a functional service provider becomes a practical, strategic partner: not a replacement for your internal team, but an extension of it that brings experience, flexibility and scalability to clinical trial execution.
In this blog, we explain what a functional service provider is, how the functional service provider model works and why this approach is often the best way for resource-constrained biotechs and pharma companies looking to advance clinical research cost-effectively.
What is a functional service provider?
A functional service provider (FSP) is a specialised outsourcing partner that supplies dedicated teams or individuals to perform one or more discrete functions of a clinical development programme — for example, data management, biostatistics, clinical monitoring, medical writing or pharmacovigilance. Unlike full-service outsourcing, the FSP focuses on specific, repeatable functions while allowing you to retain oversight and control of the clinical study strategy and decision-making.
If you’re wondering what a functional service provider is versus a traditional clinical research organisation, think of the difference as modular versus end-to-end. Rather than taking over the entire programme, the FSP plugs into the parts that need scale or specialist skills while your core team maintains overall control.
How does the functional service provider model work?
The functional service provider model typically assigns full-time equivalents (FTEs) or deliverable-based teams to work under your processes or directly within your systems. These teams behave as an extension of your staff — using your standard operating procedures (SOPs), electronic data capture (EDC) and governance structure — which simplifies data integration, maintains consistency and reduces onboarding friction across multiple studies.
Contracts can be scoped for a single study, a portfolio of programmes or for ongoing functional needs.
What are the key advantages of using a functional service provider?
Outsourcing functions to a functional service provider is a strategic choice that delivers tangible operational and financial benefits to small to mid-sized biotechs and pharma companies:
- Cost efficiency without sacrificing quality. FSP arrangements let you convert fixed headcount costs into flexible resourcing, paying only for the expertise you need when you need it.
- Rapid access to deep expertise. Whether you need experienced medical writers or pharmacovigilance specialists, FSP partners bring subject-matter experts who’ve worked across sponsors and therapeutic areas. That specialist strength accelerates timelines and reduces rework.
- Scalability and predictability. Clinical trials ebb and flow; the functional service provider model lets you scale teams up and down with predictable commercial models (FTE, per-deliverable or hybrid), so your operations stay aligned with study demands.
- Sponsor control and data stewardship. Because FSP teams often operate within sponsor systems and SOPs, you keep oversight of critical decisions and data — useful if you want to retain strategic control while offloading execution.
- Faster programme continuity and lower recruiting overhead. Hiring and retaining niche clinical staff is costly and slow. An FSP removes that recruitment burden and reduces risk from staff turnover during long, complex clinical studies.
These benefits add up: for many small biotech and pharma companies, a functional service provider is the most cost-effective path to build credible, regulatory-ready clinical operations without the long lead time and expense of creating full in-house teams.
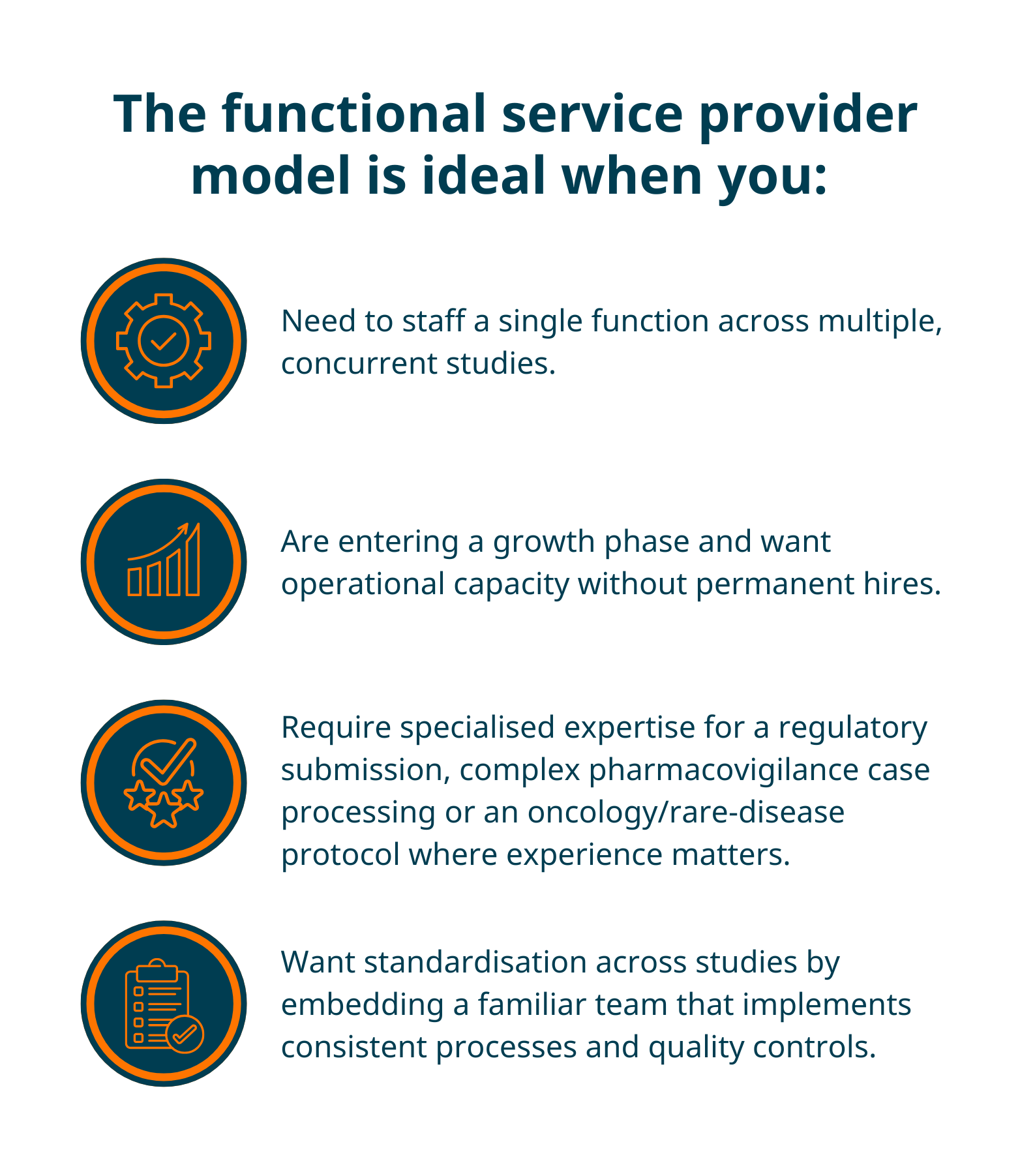
When should you consider outsourcing to a functional service provider?
Pharma consulting across all stages of your product lifecycle
Functional service provider engagements combine hands-on execution with strategic pharma consulting. For example, an FSP team delivering clinical monitoring can also feed operational insights back to programme leads, helping to optimise site selection and site performance.
This blended value is why many sponsors pair FSP resourcing with targeted pharma consulting to refine protocol design, risk-based monitoring strategy or regulatory submission readiness.
TMC Consulting provides expert-led cross-functional integration across all stages of your product lifecycle — spanning the disciplines of regulatory support, clinical development, pharmacovigilance, medical affairs and quality management.
Our experienced specialist team partners flexibly with you to guide you through complex decisions, mitigate risk and align your programme with regulatory and commercial objectives. Through extensive expertise, robust planning and flawless execution, we help you hit your critical drug development milestones compliantly, on time and within budget.
Contact our team today at connect@tmcpharma.com to learn more about our pharma consulting services and how we can help you accelerate innovative treatment access for patients who need them most.
