Pharmacovigilance (PV) plays a crucial role in assuring patient safety through the detection, evaluation, understanding and prevention of serious adverse events (SAEs) and other drug-related problems. This is why pharmacovigilance inspections and audits are so important. But what’s the difference between an audit and an inspection in pharmacovigilance? Distinguishing between an audit and an inspection is crucial for maintaining compliance and ensuring patient safety. While both are essential for evaluating PV systems, they serve different purposes:
- Pharmacovigilance audits are internal or third-party evaluations conducted by the marketing authorisation holder or sponsor to assess the effectiveness and compliance of their PV system. These proactive reviews aim to identify and rectify issues before they escalate.
- Pharmacovigilance inspections are formal assessments carried out by regulatory authorities — such as the US Food and Drug Administration (FDA), European Medicines Agency (EMA) or UK Medicines and Healthcare products Regulatory Agency (MHRA) — to verify that a company is adhering to legal obligations. These pharmacovigilance inspections vary in nature and may be unannounced and can result in significant consequences if non-compliance is found.
A successful inspection demonstrates that your PV system is robust, compliant and capable of protecting public health. Conversely, failing to meet inspection standards can lead to critical findings, warning letters, reputational damage or even product licence suspensions. In this blog, we’ll explore the 10 most common pharmacovigilance inspection findings and discuss their implications for your PV operations.
What regulators look for: common pharmacovigilance inspection findings explained
1. Maintenance of reference safety information (RSI)
Regulatory authorities frequently identify critical deficiencies related to the maintenance of RSI. These deficiencies often involve delays in submitting safety variations to update the safety sections of the summary of product characteristics and patient information leaflets. Such delays can lead to patients and healthcare professionals not receiving updated safety information promptly, potentially compromising patient safety.
2. Signal management
Critical findings in signal management are common, particularly failures to conduct signal detection activities, incorporate all available data into signal detection and delays in completing signal evaluations. These issues can result in new safety signals being identified late, affecting the timely update of product information and potentially impacting patient safety.
3. Quality management system (QMS) deficiencies
The QMS is often found lacking, with issues such as inadequate pharmacovigilance audit and deviation management, corrective and preventive action (CAPA) management, and pharmacovigilance system master file (PSMF) management. A robust QMS is essential for ensuring compliance and effective PV operations.
4. Ongoing safety evaluation
Deficiencies in ongoing safety evaluation processes are prevalent, including periodic safety update reports (PSURs) or periodic benefit-risk evaluation reports (PBRERs), inadequate assessment of adverse drug reactions (ADRs) and failure to implement risk minimisation measures (RMMs). These shortcomings can lead to delayed identification of safety concerns and inadequate risk mitigation strategies.
5. Case processing and reporting delays
Delays in individual case study reporting (ICSR) and incomplete or inaccurate data entry into electronic data capture systems are frequently observed. Such delays can hinder timely risk assessment and regulatory reporting, potentially compromising patient safety.
6. Training and competency gaps
Insufficient training and documentation of competencies for staff involved in PV activities are common findings. Lack of proper training can lead to errors in case processing, signal detection and risk management, affecting the overall effectiveness of the PV system.
7. Inadequate literature monitoring
Deficiencies in literature monitoring are often identified, such as inadequate search strategies and failure to collect and collate ADRs from literature sources. These issues can result in missing safety information and incomplete safety profiles for medicinal products.
8. Non-compliance with risk management plans (RMPs)
Failure to implement additional RMMs as outlined in RMPs is a significant concern. Non-compliance can lead to inadequate communication of risks to healthcare professionals and patients, potentially affecting patient safety.
9. Oversight and governance issues
Lack of oversight and governance, such as the absence of a qualified person for pharmacovigilance (QPPV) or inadequate access to PV records, are critical pharmacovigilance inspection findings. These issues can undermine the effectiveness of the PV system and hinder regulatory compliance.
10. Non-interventional programmes oversight
Deficiencies in the oversight of non-interventional programmes, including patient support programmes and market research programmes, are commonly identified. Issues include lack of global policies, inadequate procedures for collecting ADRs and insufficient oversight of third-party vendors. Such deficiencies can result in missing safety information from these programmes.
How to ensure compliance and patient safety with good pharmacovigilance practices (GVP)
Understanding these pharmacovigilance inspection findings and their implications can help you proactively address potential deficiencies, ensuring the safety and efficacy of your product.
Each of these findings can have significant implications for patient safety and regulatory compliance. Critical deficiencies, such as those related to RSI maintenance and signal management, can lead to delayed identification and communication of safety concerns, potentially compromising patient safety.
Major findings, including QMS deficiencies and ongoing safety evaluation issues, can undermine the effectiveness of the PV system and hinder timely risk mitigation efforts. Minor findings, while less severe, can indicate areas for improvement and should not be overlooked. Addressing these findings through corrective and preventive actions is essential for maintaining a robust and compliant PV system.
To ensure you’re always prepared for a pharmacovigilance inspection, consider maintaining an ‘inspection pack’ with key documents (PSMF, RMPs, PSURs/PBRERs, training records, PV agreements) and engage senior management in periodic reviews of CAPAs and metrics.
It’s also important to rehearse mock pharmacovigilance inspections, focusing on high-risk areas — such as third-party ICSR pathways — or effectiveness checks for risk minimisation measures.
Follow this pharmacovigilance inspection checklist to ensure success during future inspections:
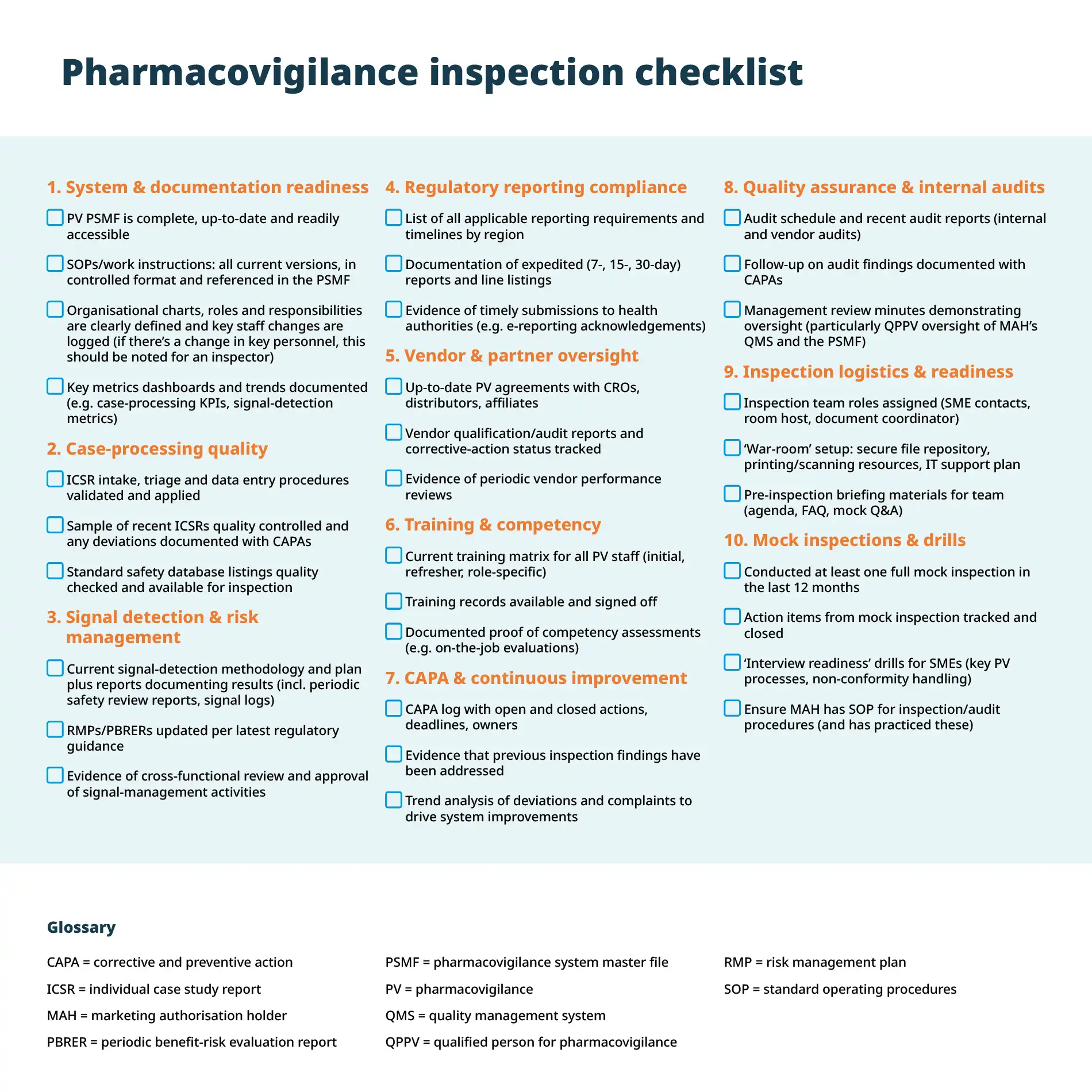
By working through this pharmacovigilance inspection checklist systematically — assigning clear ownership for each checklist item with deadlines — you’ll minimise surprises, strengthen your PV system’s robustness and ensure that you’re always ready for the next inspection.
TMC’s proven pharmacovigilance services
As a specialist global pharma services company, TMC helps you overcome common pharmacovigilance challenges and has proven success in service delivery and pharmacovigilance audits and inspections.
A recent example highlighting the strength of our PV system comes from an audit conducted by one of our biotech clients. This remote, three-day intensive audit focused specifically on our post-marketing pharmacovigilance capabilities. By adhering to the comprehensive checklist protocol outlined above, we ensured a smooth and efficient pharmacovigilance audit process — demonstrating that our PV system is not only robust and compliant but also aligned with the highest standards for safeguarding public health.
A key area of focus for the auditors was our strategic integration of AI and automation within the PV workflow — particularly how these technologies enhance signal detection, case processing and reporting efficiency. Equally important was their evaluation of our human oversight: the expertise and judgment applied to interpret, validate and act on automated outputs. This synergy between advanced technology and experienced professionals was a highlight of the audit. During the closing meeting, the auditor confirmed there were ‘no critical or major findings’ and commended our team for its professionalism, transparency and collaborative approach throughout the process.
If you need pharmacovigilance consultancy, TMC’s end-to-end pharmacovigilance services help you apply these strategies seamlessly. From QPPV provision and PSMF governance to advanced signal detection, clear regulatory communications and meticulous document management, we ensure you meet UK, EU, US and global PV requirements and comply with international pharmacovigilance standards.
Find out more about TMC Consulting or contact our team today at connect@tmcpharma.com to see how we can help you overcome common PV challenges and ensure patient safety in therapeutic treatments.
