Your guide to addressing the 6 most common pharmacovigilance challenges for MAHs
Effective pharmacovigilance (PV) is vital to safeguarding patients, especially in rare disease therapies where limited clinical trial and post-market data heighten uncertainty.
The rarity of these conditions means that each individual safety report carries significant weight; patient cohorts are small and data-scarce, meaning rigorous PV is critical in identifying safety signals early to safeguard vulnerable patients.
However, maintaining Good Pharmacovigilance Practices (GVP) involves adhering to a complex spectrum of global regulations — including Pharmacovigilance System Master File (PSMF) requirements, creating comprehensive Risk Management Plans (RMPs) and producing timely Periodic Safety Update Reports (PSURs) or Periodic Benefit-Risk Evaluation Reports (PBRERs). All these requirements pose unique challenges for organisations with limited PV experience.
In this guide, we'll cover six pharmacovigilance challenges for marketing authorisation holders (MAH) and the solutions to these common problems, offering insights on when to engage specialist pharmacovigilance services or a pharmacovigilance consultancy and practical advice on:
- Interpreting intricate PV regulations and mapping obligations.
- Harmonising compliance across the UK, EU, US, China, Japan and beyond.
- Building and maintaining a robust PSMF.
- Applying advanced signal detection to limited datasets.
- Ensuring transparent, timely communications with regulators and patients.
- Managing key documents, such as RMPs, PSURs and PBRERs.
Contents:
1. Navigating complex pharmacovigilance compliance requirements
2. Understanding differing rules across regions and countries
3. Maintaining robust pharmacovigilance systems and procedures
4. Analysing and reporting with limited clinical safety data
5. Establishing clear communication with regulators and the public
6. Managing challenging pharmacovigilance documents
Ensuring patient safety with Good Pharmacovigilance Practices
1. Navigating complex pharmacovigilance compliance requirements
The first challenge is understanding and operationalising the myriad pharmacovigilance regulations that apply equally to rare disease therapies despite their small safety datasets.
Smaller pharmaceutical or biotech companies often find it overwhelming to translate high-level guidance — such as the European Medicines Agency's (EMA) GVP modules or the US Food and Drug Administration's (FDA) 21 CFR Part 314 requirements — into day-to-day workflows. This can leave gaps in Individual Case Safety Report (ICSR) timelines, signal detection processes and periodic reporting schedules. Without a clear roadmap, responsibilities blur between clinical, medical and regulatory teams, increasing the risk of late reports or incomplete submissions.
To address these PV compliance challenges, you should:
- Map obligations to workflows. Create a detailed matrix linking each requirement — ICSR timelines, signal detection thresholds, PSURs/PBRERs, submission windows — to your Standard Operating Procedures (SOPs) and clinical operations. This ensures no deadline is overlooked and that responsibilities (e.g. case processing versus medical review) are clearly assigned.
- Leverage modular GVP guidance. Break down the modules of EMA's GVP into operational checklists (e.g. Module II for PSMF and Module V for signal detection) to simplify adoption and audits.
- Automate routine tasks. To reduce manual effort and non-compliance risk, use safety database tools that auto-fill ICSR data fields, assist in highlighting reporting timelines, generate templated line listings for ongoing safety evaluation activities (e.g. PSURs, signal detection activities) and monitor risk management and minimisation commitments.
- Train cross-functional teams. Regular workshops for clinical, medical and regulatory staff help create a shared understanding of PV concepts and GVP, strengthening the entire safety ecosystem. Periodic internal pharmacovigilance audits will also help identify gaps and provide opportunities for process improvement before regulatory pharmacovigilance inspections.
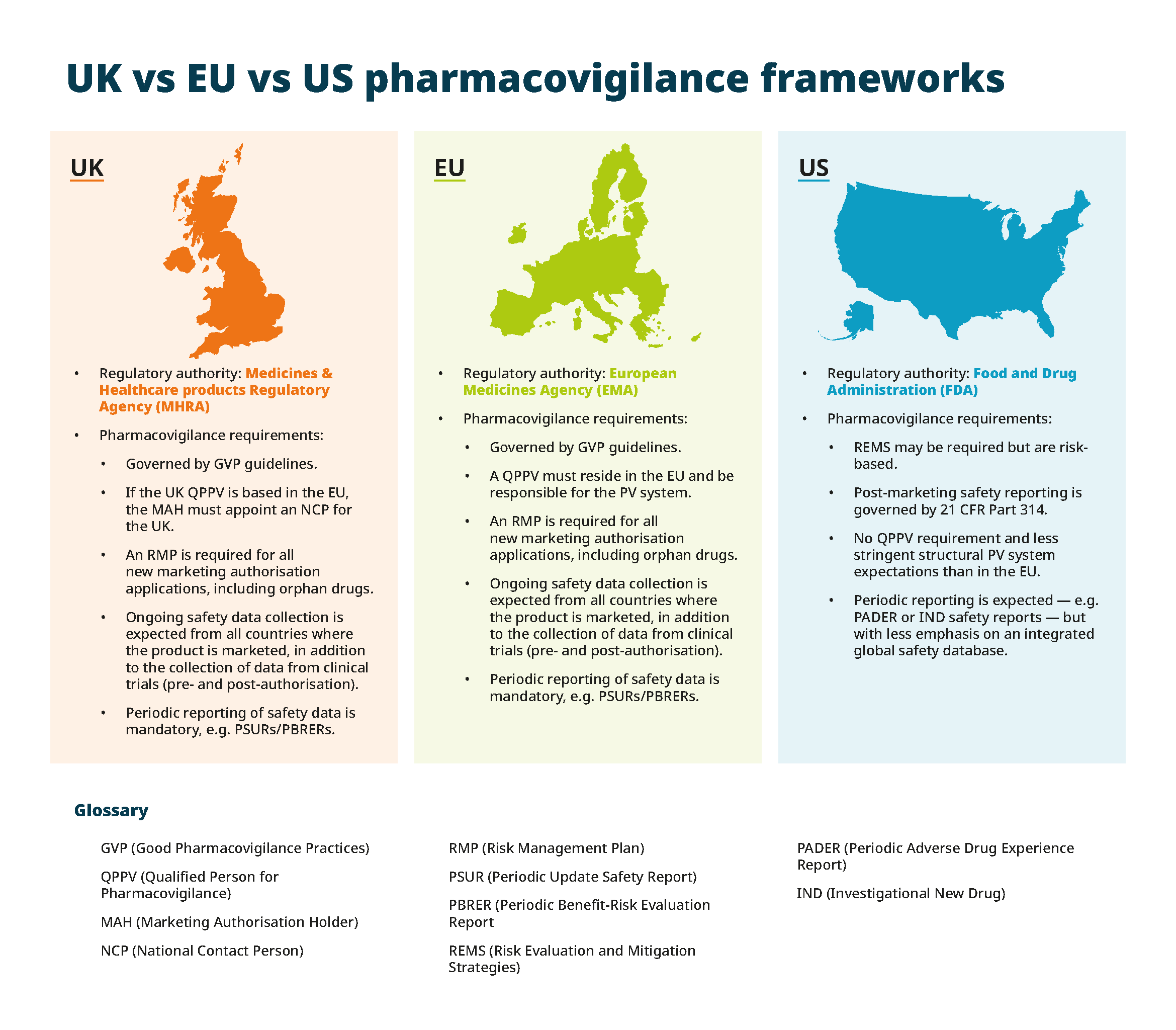
2. Understanding differing rules across regions and countries
Rare disease MAHs face a second major hurdle in reconciling divergent pharmacovigilance frameworks across jurisdictions.
Although the EU operates a unified PV framework via the EMA, individual Member States may impose country-specific conditions — creating a patchwork of requirements. In contrast, the US FDA's post-marketing safety reporting under 21 CFR 314 focuses on risk-based approaches like Risk Evaluation and Mitigation Strategies (REMS) and periodic reports.
If you hold licences or market a drug outside the UK and EU, such as in China, and later apply for an EU marketing authorisation, UK and EU regulators expect full transparency regarding global safety data. For example, depending on the nature of the case, the regulators may require the MAH to report non-domestic ICSRs to their respective vigilance systems (e.g. EudraVigilance), and any signals from these markets will inform the maintenance of the UK and EU RMP and PSMF.
When to appoint a Qualified Person for Pharmacovigilance (QPPV)
Appointing experienced local PV specialists under a global Qualified Person for Pharmacovigilance (QPPV) is one manner in which MAHs can maintain (and in some countries are required to) their PV compliance.
In the UK and EU, the QPPV has oversight and independent control over the MAH's quality management system (QMS) and pharmacovigilance system. They are responsible for ensuring the MAH maintains compliance, conducting comprehensive safety monitoring and evaluation activities, and liaising with authorities on all safety matters. The QPPV role is also essential to protect public health and maintain communication with healthcare providers and regulators.
Appointing a QPPV is mandatory in the UK and EU. Local PV experts manage national PV activities, and the global QPPV oversees the integration of safety data from across the region to ensure pharmacovigilance services meet both UK and EU regulatory requirements.
Although there's no requirement for the EU-style QPPV in the US, this doesn't reduce the burden on companies to uphold high pharmacovigilance standards. In the US, the MAH must ensure they have the relevant expertise to ensure compliance with FDA expectations, which may be a challenge for small teams.
3. Maintaining robust pharmacovigilance systems and procedures
A third challenge is sustaining a reliable PV infrastructure that evolves with organisational changes and regulatory updates. The PSMF must accurately reflect governance, SOPs and performance metrics; failure to update it promptly can lead to non-compliance findings during inspections.
In addition, without defined KPIs — such as median ICSR processing time, PSURs/PBRERs, submission timelines and signal management activities — you'll lack visibility into system performance, hindering your ability to drive continuous improvement.
A well-designed pharmacovigilance system not only meets regulatory standards but also supports continuous improvement. So, to overcome this challenge, you must consider:
- PSMF governance. Assign a dedicated owner for the PSMF who coordinates regular reviews, tracks change logs and liaises with quality assurance and regulatory teams.
- KPI-driven monitoring. Define metrics (e.g. median ICSR processing time, PSURs/PBRERs, submission timeliness, signal detection activities) and review them monthly to identify trends and implement corrective actions.
- Routine PV audits. Conduct internal pharmacovigilance audits to evaluate SOP adherence, database integrity and training effectiveness. Focus on high-risk areas — such as third-party ICSR reporting pathways — or effectiveness checks for risk minimisation measures.
- Inspection readiness. Maintain an 'inspection pack' with key documents — PSMF, RMPs, PSURs/PBRERs, training records, PV agreements — and rehearse mock pharmacovigilance inspections to build team confidence.
4. Analysing and reporting with limited clinical safety data
Small patient populations limit the quantity and diversity of safety data, making traditional disproportionality analyses less reliable and hindering the detection of rare adverse events. Rare events may not trigger statistical thresholds, and clinical significance can be obscured without contextual expertise. This scientific challenge can delay signal identification and risk mitigation, posing a risk to patient safety.
When safety datasets are limited, combining quantitative and qualitative methods can be a beneficial approach. Rigorous methodologies and cross-disciplinary expertise are essential. These methodologies and expertise include:
- Qualitative signal detection. Apply a structured, multi-step process (case identification, medical review, literature cross-check, expert panel assessment) to uncover subtle signals in small datasets.
- The use of external data. Leverage disease registries, real-world evidence and patient advocacy networks to supplement clinical trial data and post-marketing experience, expanding the evidence base for safety assessments.
- Adaptive threshold setting. For rare events, adjust disproportionality analysis parameters to balance sensitivity and false-positive rates based on expert-driven criteria.
- Interdisciplinary reviews. Convene PV, clinical, statistical and regulatory experts in periodic safety review meetings to interpret findings, prioritise signals and plan risk-minimisation measures. All safety trends — no matter how tentative — should feed into PSURs and PBRERs to inform RMP revisions and risk-minimisation strategies.
5. Establishing clear communication with regulators and the public
Maintaining a transparent, accurate and timely dialogue with health authorities, healthcare professionals and patients is essential for trust but is often undervalued by resource-constrained teams. Misaligned messaging or delayed submissions of Urgent Safety Communications (USC) can lead to regulatory sanctions and erode public confidence.
Transparent, compliant messaging builds trust and meets regulatory expectations, so you should focus on the following:
- Regulatory updates. Use standardised templates for USC and periodic reports, ensuring consistency in format, terminology and data presentation.
- Patient-centric materials. Develop plain-language summaries of PBRER findings and PSUR highlights for patient and healthcare provider audiences, following relevant regulatory authority guidance on readability and risk communication.
- Stakeholder timelines. Maintain a stakeholder calendar that maps each submission and communication deliverable to avoid last-minute rushes. This also allows alignment across functions, reducing confusion regarding key ongoing safety measures/findings.
- Crisis communication plan. Draft a rapid-response protocol for safety signals requiring media engagement and notifying regulators and healthcare providers — with pre-approved spokespeople, key messages and escalation pathways.
6. Managing challenging pharmacovigilance documents
The sixth challenge is the sheer volume and complexity of pharmacovigilance documentation required during development and post-approval.
Biotech and pharmaceutical companies must produce PSURs at defined frequencies, update RMPs as new signals arise, compile PBRERs and implement Risk Minimisation and Mitigation Strategies (RMMS), all tailored to regional templates.
Efficient document management is crucial to meet diverse regional requirements and timelines, so you should implement the following:
- A centralised repository. Host all PV documents — PSURs, PBRERs, RMPs, RMMS — in a validated document management system with role-based access and audit trails; system backup and archiving considerations must not be overlooked.
- Version control and templates. Use region-specific templates (e.g. the EU's integrated RMP format) and enforce strict version numbering to avoid submission errors. This is particularly important where regional differences may occur between documents (e.g. adverse reactions listed within the EU SmPC vs. USPI), necessitating careful management and communication of document updates between functions. In this example, the creation and maintenance of a Company Core Data Sheet (CCDS) adds clarity during periodic safety report drafting.
- Regular cross-checks. Schedule regular cross-document reviews, ensuring RMP risks align with PSUR findings and PBRER sections reflect current signal evaluations.
Additionally, understanding the difference between audit and inspection in pharmacovigilance —where audits are internal or partner-led assessments and inspections are regulator-led systems and compliance investigations — is crucial to preparing appropriate evidence and responses.
Ensuring patient safety with good pharmacovigilance practices Ensuring patient safety with good pharmacovigilance practices
Addressing these six common challenges requires both in-depth pharmacovigilance expertise and practical, adaptable systems. By applying the detailed strategies outlined here, you can strengthen your safety frameworks and demonstrate robust patient-risk management.
TMC's expertise as a specialist global Clinical Research Organisation (CRO) and pharmacovigilance consultancy helps you apply these strategies seamlessly. By providing end-to-end pharmacovigilance services — from QPPV provision and PSMF governance to advanced signal detection, clear regulatory communications and meticulous document management — we ensure you meet UK, EU, US and global PV requirements and comply with international pharmacovigilance standards.
Through tailored systems, expert teams and proven success in service delivery and pharmacovigilance audits and inspections, we ensure that patient safety remains the top priority in even the most challenging rare disease programmes.
Find out more about TMC's pharmacovigilance services or contact our team today at info@tmcpharma.com to see how we can help you overcome these PV challenges and ensure patient safety in rare disease therapies.
