Your ultimate guide to appointing a marketing authorisation holder
Biotech and pharmaceutical companies face substantial regulatory hurdles in bringing new therapies and products to market, particularly when it comes to rare diseases. The marketing authorisation holder (MAH) function is critical to helping you navigate these challenges successfully.
In this guide, we’ll explain the strategic role of a marketing authorisation holder in managing every phase of drug development — from initial regulatory submissions and clinical trial oversight to post-approval regulatory affairs — to ensure your products remain safe, effective and compliant throughout their life cycle.
Contents:
- Defining the marketing authorisation holder role
- Understanding the different types of regulatory submissions
- Clinical trial application (CTA)
- 5 steps to a successful clinical trial application
- Market authorisation application (MAA)
- 5 steps to a successful market authorisation application (MAA)
- Other key regulatory submissions: IND, NDA and BLA
- Comparing regional regulatory submission frameworks
- The UK/EU framework
- The US regulatory landscape
- Navigating the challenge of post-approval regulatory affairs
- Challenge 1: periodic safety update reports and pharmacovigilance
- Challenge 2: compliance with evolving regulatory requirements
- Challenge 3: serious adverse event reporting and risk management
- Challenge 4: managing ongoing clinical trials
- Challenge 5: handling the regulatory submission process for variations
- Strategies for maximising market potential
- Key factors for a successful partnership
- Ensuring regulatory compliance and market success
-
Defining the marketing authorisation holder role
Defining the marketing authorisation holder role
Key aspects of the role include:
- End-to-end compliance management and regulatory accountability. The marketing authorisation holder ensures all facets of the product’s lifecycle — from pre-market research through to post-approval regulatory affairs — are handled in accordance with the latest regulatory guidelines. They must maintain detailed documentation, manage risk assessment and mitigation, and remain compliant with changing regulatory guidelines.
- Clinical and non-clinical oversight. This includes preparing and submitting clinical trial applications (CTAs) to initiate clinical studies, followed by the compilation of comprehensive dossiers — such as marketing authorisation applications (MAAs) or new drug applications (NDAs) — to gain market access.
- Liaison with regulatory authorities. The marketing authorisation holder serves as the main point of communication with agencies (such as the EMA, MHRA or FDA), which is fundamental for clarifying data requirements, addressing queries and ensuring updates or amendments are properly managed.
- Continuous monitoring. The role extends beyond initial product approval to include continuous safety surveillance and the management of post-approval regulatory affairs, ensuring any emerging safety concerns are addressed promptly.
This role is critical in ensuring products consistently meet the stringent safety, efficacy and quality standards required by regulatory authorities.
2. Understanding the different types of regulatory submissions
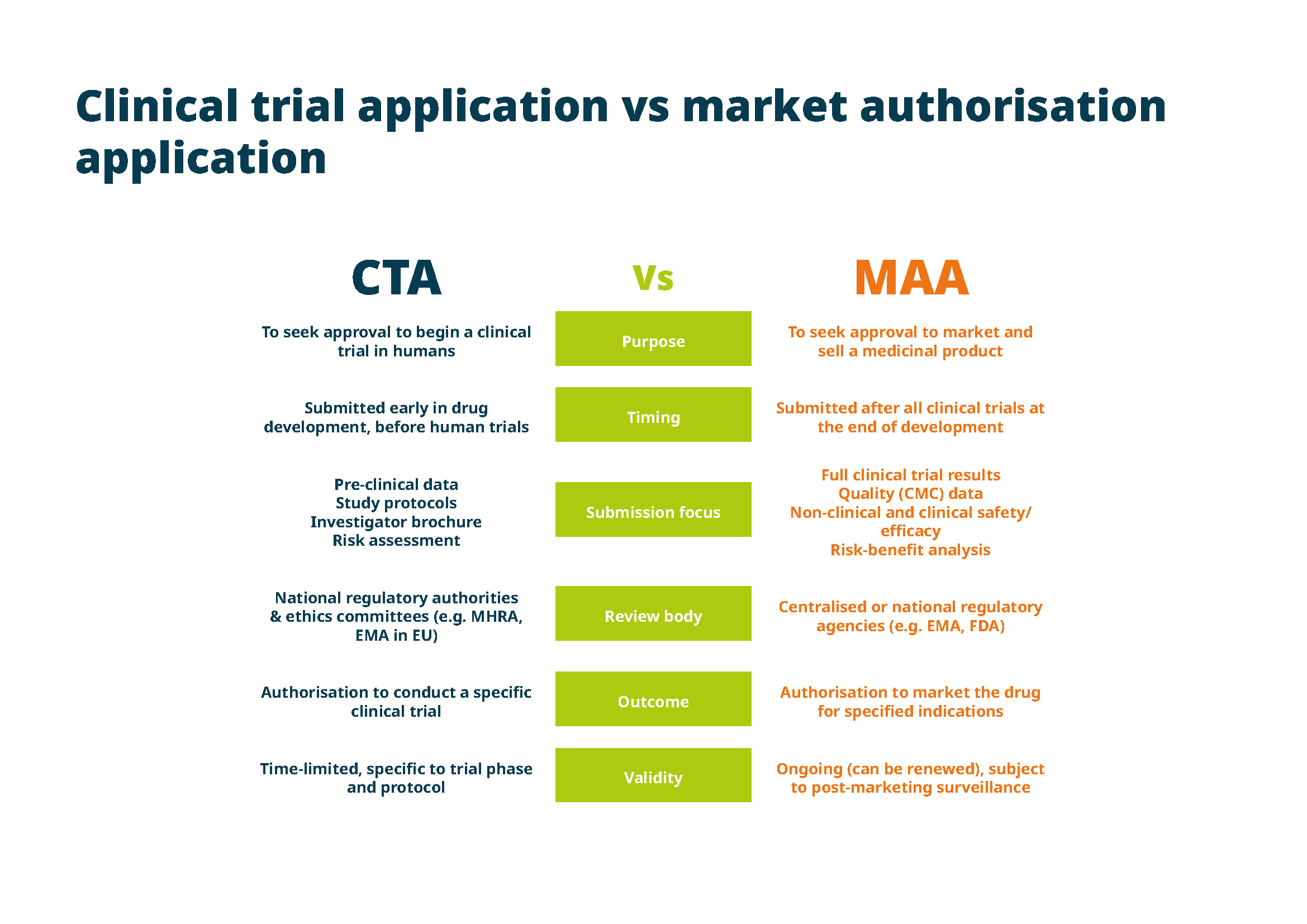
The regulatory submission process is an intricate pathway that every biotech and pharmaceutical product must traverse. Two of the most significant types of regulatory submissions you’ll need to navigate throughout the life cycle of a drug are clinical trial applications and marketing authorisation applications.
Clinical trial application (CTA)
A clinical trial application is one of the first regulatory submissions you’ll make during the drug development process. The CTA is submitted to the relevant regulatory authority before initiating a clinical trial. Its purpose is to gain approval to conduct human trials for a new investigational drug or device.
In the EU, CTAs are submitted to national regulatory authorities or the EMA if the trial is to be conducted in multiple EU member states. A CTA typically includes:
• Detailed descriptions of the planned clinical studies, including methodologies and endpoints.
• Strategies for monitoring patient safety during the trial, including adverse event tracking.
• Ethical considerations to ensure the trial design adheres to ethical standards and receives the necessary approvals.
The clinical trial application must be carefully prepared to meet regulatory requirements, as approval is needed before any human trials can begin. If the CTA isn’t approved, the clinical trial cannot proceed.
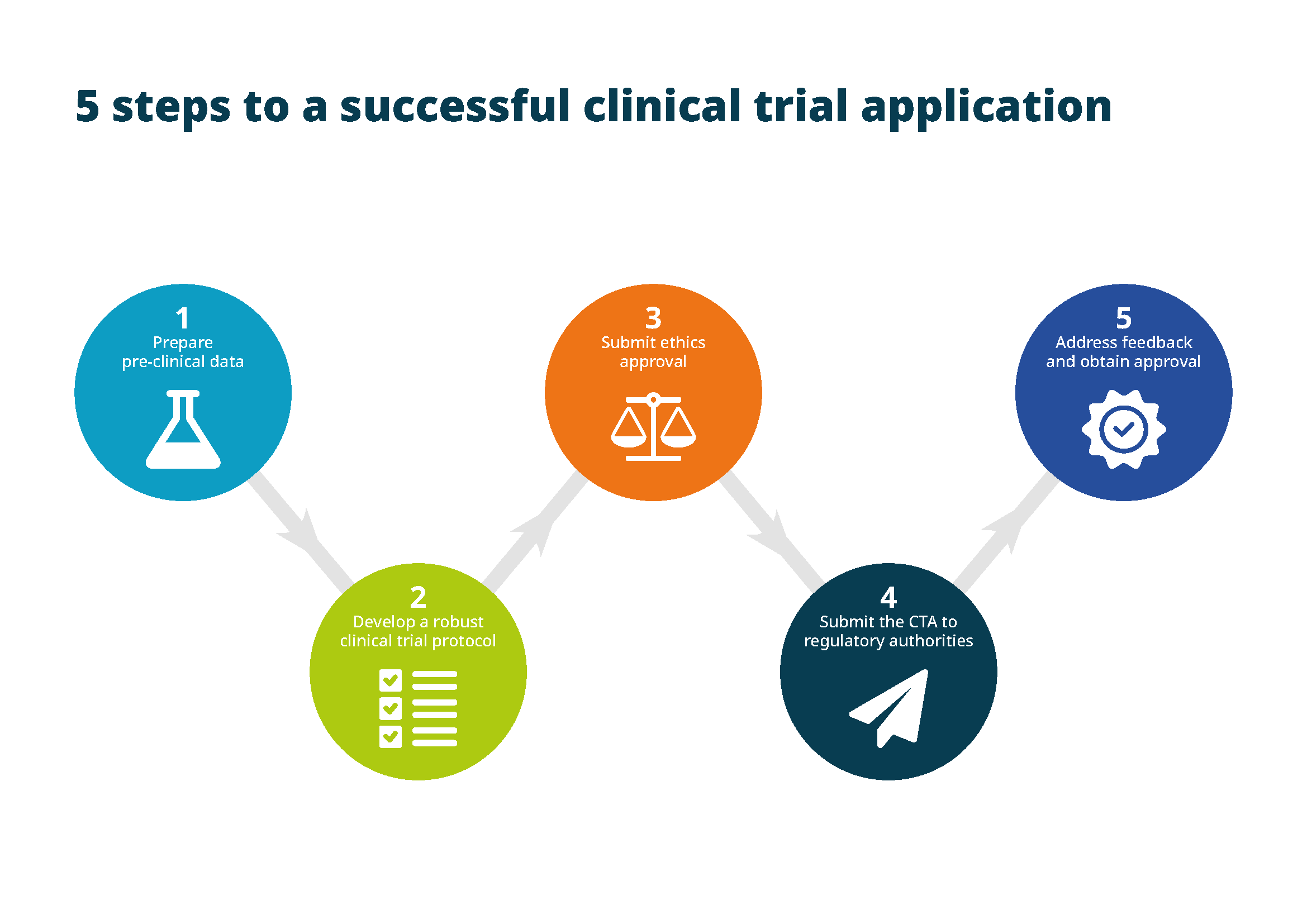
To achieve a successful clinical trial application, you need to follow five key steps:
- Prepare pre-clinical data. You must gather extensive pre-clinical data from laboratory and animal studies before submitting a CTA. This data should demonstrate the drug’s safety profile, including its potential for toxicity or side effects, as well as its mechanism of action.
- Develop a robust clinical trial protocol. The protocol must include detailed plans for study design, inclusion and exclusion criteria for participants, endpoints (primary and secondary) and methods for data collection. It must also align with international guidelines, such as good clinical practice (GCP), and demonstrate a clear pathway for patient safety.
- Submit ethics approval. Ethical approval is required before any clinical trial can proceed. An independent ethics committee or institutional review board must review your trial’s design, the informed consent process and the safety precautions taken for participants.
- Submit the CTA to regulatory authorities. The CTA, complete with all necessary documentation, is then submitted to the regulatory authorities. In the EU, this can be done to either national authorities or the EMA, depending on the scope of the clinical trial.
- Address feedback and obtain approval. Regulatory authorities will review your clinical trial application and may request additional data or modifications to the study protocol. Addressing their feedback promptly and thoroughly is key to obtaining approval and moving forward with the clinical trial.
An experienced marketing authorisation holder can minimise delays and reduce the risk of rejection by advising you and helping you submit a clinical trial application that meets regulatory requirements.
Market authorisation application (MAA)
Once clinical trials have demonstrated that a drug is safe and effective, you must compile and submit a comprehensive dossier for final marketing approval. In the EU, the MAA is also submitted to the EMA (or to national regulatory authorities if the drug will be marketed only in a specific country).
The marketing authorisation application is a critical step toward commercialisation, as approval will allow you to market and sell the drug within the EU. However, approval can be complex, and regulatory authorities may request additional information or clarification before granting authorisation.
An MAA regulatory submission includes a comprehensive package of data collected during the drug development process, including:
- Summaries and detailed reports of all pre-clinical and clinical findings to substantiate safety and efficacy.
- Details on the drug’s formulation, production methods, quality control processes and compliance with Good Manufacturing Practices (GMP).
- Proposed labelling information, including dosage instructions, indications, side effects and other relevant details.
- Documents that outline strategies for the ongoing monitoring of product safety, such as pharmacovigilance and risk management plans (RMP), to ensure readiness for any necessary amendments or variations following product launch.
Additional submissions may also include:
- Adjustments to the original submission to reflect product or process modifications.
- Periodic safety update reports (PSURs) that help maintain vigilance over the product’s performance in the market.
- Renewal applications for the periodic re-evaluation of market authorisation.
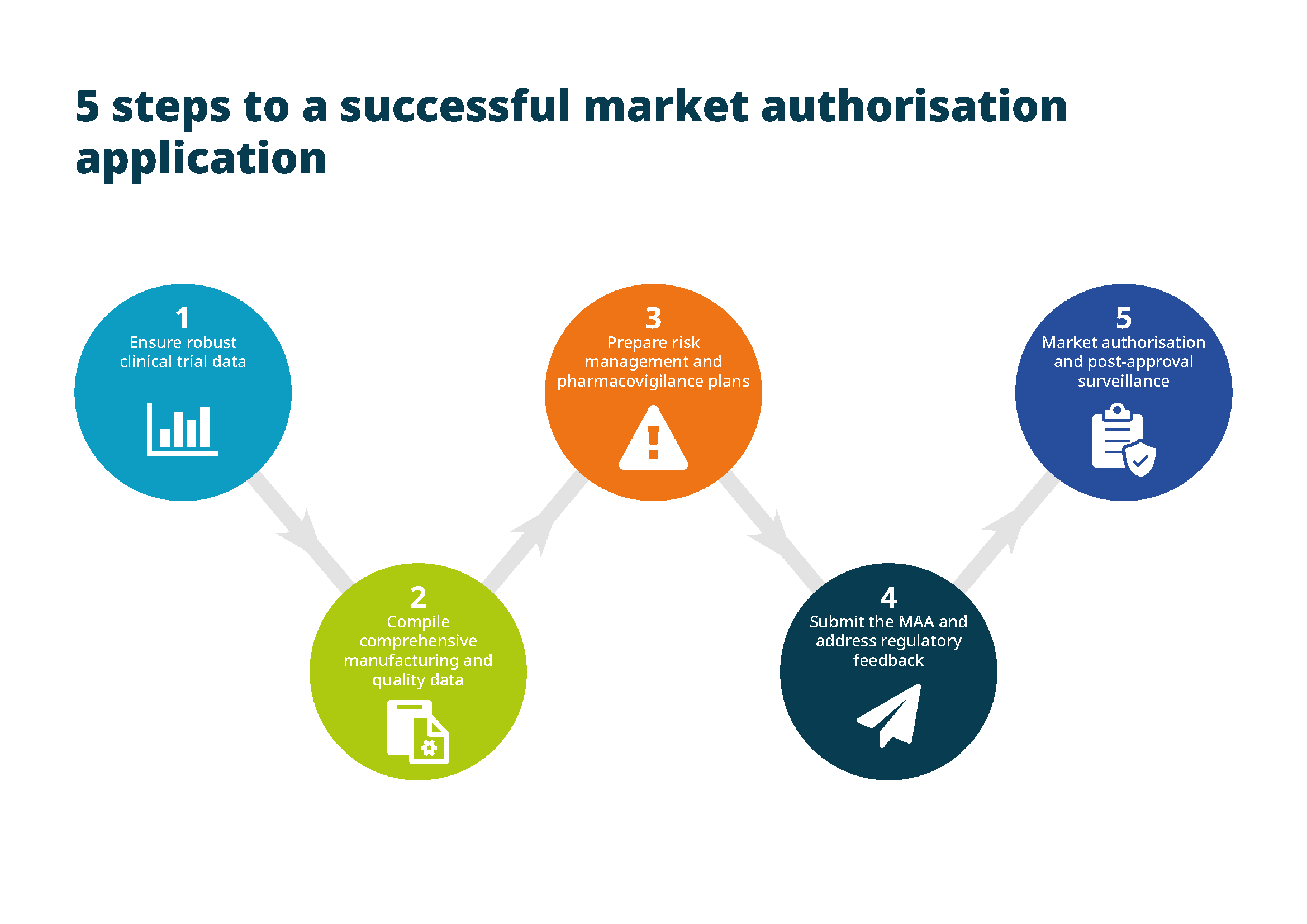
For a successful marketing authorisation application, you need to follow five key steps:
- Ensure robust clinical trial data. Before submitting an MAA, you must have completed the required trials demonstrating the safety and efficacy of the drug. The results must be statistically significant and show a favourable benefit-risk profile.
- Compile comprehensive manufacturing and quality data. The MAA must include information about the drug’s formulation, manufacturing processes and quality control measures. Regulatory authorities will assess whether the product is consistently manufactured to meet quality standards.
- Prepare risk management and pharmacovigilance plans. The MAA regulatory submission must include detailed plans for monitoring the drug’s safety once it’s on the market. These plans should outline how you’ll report adverse events and manage ongoing safety.
- Submit the MAA and address regulatory feedback. Once you’ve submitted the marketing authorisation application, regulatory authorities will review it and may request additional data or clarification. Responding to these requests in a timely and thorough manner is critical to obtaining approval.
- Market authorisation and post-approval surveillance. Upon receiving approval, you can market the drug within the EU. However, post-approval surveillance will continue to monitor the safety and efficacy of the drug in the broader patient population.
A marketing authorisation holder can advise you and help you compile the necessary data and documents to ensure a successful submission.
Other key regulatory submissions: IND, NDA and BLA
Different regions have varying requirements for regulatory submissions. While the CTA and MAA are the primary types of regulatory submissions in the UK and EU, other submissions may be necessary depending on the jurisdiction.
For example, in the US, you must submit an investigational new drug (IND) application to the FDA before conducting clinical trials. The IND includes data similar to that of a CTA but is tailored to meet the FDA’s requirements.
A new drug application (NDA) is the US equivalent of the MAA and is submitted to the FDA once clinical trials are complete. The NDA contains data on the safety, efficacy and quality of the drug. This is necessary for market approval.
A biologics license application (BLA) is similar to an NDA but is used specifically for biological products, such as vaccines or gene therapies.
3. Comparing regional regulatory submission frameworks
As mentioned in the previous section, different regions have varying requirements for regulatory submissions. The role of a marketing authorisation holder in the UK/EU may, therefore, differ slightly from that of a marketing authorisation holder in the US. For example:
- In the UK/EU, the process is highly structured, with an emphasis on detailed risk management, ongoing safety monitoring and strict adherence to standardised formats.
- In the US, while the core requirements are comparable, there is often a more flexible, iterative approach, which sometimes allows for modifications in the presentation of data and incremental updates throughout the review process.
Understanding the regional differences in regulatory standards is crucial when looking at the marketing authorisation holder function.
The UK/EU framework
The regulatory environment within the UK and EU is defined by comprehensive and well-established guidelines. Important aspects include:
- Detailed documentation. Applications must include extensive information on clinical and non-clinical data, manufacturing processes and ongoing safety measures and risk management.
- Structured processes. Regulatory submission procedures are rigid and follow a centralised or decentralised review process, depending on the product type. Regulatory bodies also conduct inspections to verify adherence to quality standards and efficacy benchmarks.
- Robust risk management. Mandatory risk management plans (RMPs) and continuous pharmacovigilance systems ensure any safety issues are managed proactively.
- Ongoing compliance. Continuous obligations such as regular PSURs, RMP updates and prompt reporting of variations are required to maintain compliance after the initial approval.
The US regulatory landscape
In contrast, the US regulatory process, managed by the FDA, offers certain flexibilities along with its own set of challenges:
- Iterative submission process. The FDA often requests additional data, making the regulatory submission process more dynamic. Initial submissions are often followed by additional requests for clarification or new data.
- Emphasis on benefit-risk analysis. Presentations of clinical data are carefully scrutinised to ensure a balanced view of benefits versus potential or known risks.
- Flexible documentation formats. While rigorous, the US process allows for varying document structures that can be tailored for clarity and compliance.
- Post-approval commitments. Continuing obligations might include further clinical studies and adjustments to the product labelling as more safety data becomes available.
When looking at the marketing authorisation holder function, it’s important to understand how these requirements impact documentation preparation, timeline management and resource allocation.
Additionally, if you’re operating across multiple territories, developing a unified approach to regulatory submissions is essential. Key considerations include:
- Establishing a core dossier that can be adapted to meet specific regional requirements.
- Maintaining current knowledge of evolving regulations in each target market.
- Evaluating whether to develop internal expertise or leverage external support (this strategic decision will be influenced by the complexity and scale of your operations).
4. Navigating the challenges of post-approval regulatory affairs
Bringing a new drug to market doesn’t end with approval from regulatory authorities. Once a product is on the market, continuous oversight is required to ensure safety, efficacy and regulatory adherence.
This phase presents several challenges for biotech and pharmaceutical companies, which can be mitigated by the help of a marketing authorisation holder as their responsibilities shift from pre-approval regulatory submissions to managing ongoing post-approval regulatory affairs.
Challenge 1: periodic safety update reports and pharmacovigilance
The sheer volume of post-market data makes it incredibly difficult to track and report on a drug’s safety, especially when it comes to compiling periodic safety update reports (PSURs) that comprehensively summarise global serious adverse events.
The marketing authorisation holder’s role involves ensuring these safety updates are submitted on time and with precision. This systematic approach not only meets regulatory requirements but also enhances ongoing pharmacovigilance, thereby safeguarding patient well-being.
Challenge 2: compliance with evolving regulatory requirements
Global regulatory guidelines are in constant flux, with changes in drug approval processes and clinical trial standards creating a moving target for compliance.
A key function of the marketing authorisation holder is to continuously monitor and adapt to these regulatory shifts. By staying abreast of the latest industry standards — and, if necessary, collaborating with external regulatory specialists — they ensure all submissions, including your marketing authorisation application (MAA), are current and compliant, thereby reducing the risk of non-compliance and streamlining the approval process.
Challenge 3: serious adverse event reporting and risk management
Promptly identifying and reporting serious adverse events is critical, yet the complexity and urgency involved in this task can lead to delays or inaccuracies that jeopardise patient safety and regulatory standing.
The marketing authorisation holder is tasked with managing this challenge by establishing robust systems for real-time monitoring and risk management. By efficiently tracking adverse reactions and implementing comprehensive risk management plans, they help you ensure serious adverse events are reported accurately and in a timely manner. This proactive approach helps mitigate potential harm and protects the integrity of your drug’s safety profile.
Challenge 4: managing ongoing clinical trials
Even after a drug is approved, the need for ongoing clinical research — such as post-marketing Phase IV studies — poses a challenge in maintaining regulatory oversight and gathering reliable long-term efficacy and safety data. A marketing authorisation holder must address this challenge by orchestrating the execution of post-marketing clinical trials with rigorous regulatory oversight. This involves designing studies that adhere to stringent standards and ensuring data from these trials is accurately reported. This continuous research not only provides vital insights into the drug’s long-term performance but also reinforces its safety and effectiveness in real-world settings.
Challenge 5: handling the regulatory submission process for variations
Once a marketing authorisation has been granted, any subsequent variations — such as changes to manufacturing processes, new therapeutic indications or label modifications — must be meticulously documented and approved, a process that can be both time-consuming and complex.
The marketing authorisation holder manages these complexities by diligently preparing and coordinating all necessary documentation for variations. This involves assembling robust clinical data and ensuring each submission complies with current regulatory guidelines. By maintaining a systematic approach to these processes, you can effectively handle any updates or modifications, thus sustaining ongoing compliance throughout the product’s lifecycle.
5. Strategies for maximising market potential
For many biotech and pharmaceutical firms, investing in an in-house team solely dedicated to managing these regulatory responsibilities can be both resource-intensive and complex. So, the decision to outsource the marketing authorisation holder function is not just about reducing risks — it’s a strategic move to enhance market potential and secure long-term success.
By outsourcing your marketing authorisation holder function, you can transform regulatory challenges into opportunities for competitive advantage.
Outsourcing the marketing authorisation holder role allows you to:
- Accelerate market entry. Faster and more efficient regulatory submissions lead to quicker market approvals.
- Maximise cost efficiency. Reduce the overhead costs associated with maintaining internal regulatory teams and infrastructure.
- Enhance patient safety. Expert compliance management and ongoing safety monitoring ensure product standards remain high throughout the lifecycle.
- Increase flexibility. Scale resources up or down based on the project’s needs, ensuring efficient use of internal resources for core research and development activities.
Key factors for a successful partnership
When considering whether to manage the marketing authorisation holder function in-house or through an external partner, there are several key points to consider:
Comprehensive expertise and capabilities
Evaluate potential partners (if outsourcing) or ensure internal teams have a proven history in managing both the regulatory submission process and post-approval regulatory affairs.
It’s important that the team understands regional regulatory nuances — such as the requirements for a marketing authorisation holder in the UK/EU versus those in the US — as well as the practical challenges of ongoing regulatory management.
Cost and resource efficiency
Determine how best to allocate internal resources to core activities such as research and product development while ensuring regulatory responsibilities are met effectively. In many cases, outsourcing may prove more efficient.
You should also consider the relative costs of maintaining an in-house regulatory team versus the potential economies of scale provided by external experts.
Strategic fit and long-term objectives
The chosen approach should be flexible enough to adapt to changes in regulatory guidelines or the scope of the product portfolio.
Whether handled internally or externally, quality management systems must be robust enough to support every stage of the marketing authorisation holder function — from CTAs and MAAs to continuous post-approval regulatory affairs.
A clear framework for communication with regulatory authorities is also essential, as is having defined roles and responsibilities within your organisation or between your organisation and an external partner.
Ensuring regulatory compliance and market success
The marketing authorisation holder plays a pivotal role in successful regulatory submissions and ongoing product compliance, making them an indispensable part of the new drug approval process.
From preparing and submitting applications to overseeing post-market surveillance and drug safety, the MAH ensures your drug remains safe, effective and compliant throughout its life cycle.
The marketing authorisation holder’s responsibilities directly impact patient safety and product integrity. So, with regulatory landscapes constantly evolving, the marketing authorisation holder must be proactive in keeping up with changes and ensuring continuous compliance.
You can significantly improve your chances of gaining market authorisation approval and secure substantial discounts on EMA fees with TMC’s support for regulatory submissions. These services include acting as your marketing authorisation holder, if you don’t have a presence in Europe, to ensure compliance with all local regulations.
We take on all the legal and regulatory accountability to ensure your product is manufactured, marketed and distributed according to the terms and conditions laid out in the marketing authorisation.
As an established EU/EEA-based marketing authorisation holder with SME status, TMC can be your EU/EEA representative — simplifying new drug approvals and maximising your savings.
Find out more about TMC’s regulatory services or contact our team today at regulatory.services@tmcpharma.com to see how we can support you through the regulatory submission process and help you secure EMA new drug approvals efficiently and cost-effectively.
