Your easy guide to orphan drug designation for rare diseases
In this guide, we’ll clarify the orphan drug designation criteria for the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), outline the application process, look at the advantages and challenges of gaining orphan drug designation and discuss the practical steps you can take to secure designation.
Whether you’re initiating your first clinical trials in a niche population or refining late-stage development, this guide will give you an overview of what you need to know to achieve orphan drug status and bring your critical therapy to market.
Contents:
- What are the orphan drug designation criteria?
- FDA orphan drug designation vs EMA orphan drug designation
- What is the orphan drug designation application process?
- What are the benefits of orphan drug designation?
- Market exclusivity
- Regulatory fee incentives
- Grant funding and tax credits
- Scientific advice and protocol assistance
- Enhanced visibility and stakeholder engagement
- What are the common challenges associated with orphan drug designation?
- Challenge 1: limited patient populations
- Challenge 2: epidemiological uncertainty
- Challenge 3: diagnostic heterogeneity
- Challenge 4: evolving regulatory guidance
- Challenge 5: data gaps and resource constraints
- Challenge 6: global variability
- Challenge 7: aligning with patient advocacy groups
- How to get an orphan drug designation
- Early and continuous patient organisation engagement
- Transparent trial design and endpoint selection
- Articulate unmet needs with real-world evidence
- Make use of incentive programmes and grants
- Harmonise US and EU submissions
- Robust data packaging and continuous updates
1. What are the orphan drug designation criteria?
Before we look at the advantages and challenges, let’s start by looking at the criteria for achieving ODD and how this differs across two key markets: the EU and the US.
To meet the orphan drug designation criteria, sponsors must establish that:
- The disease meets the prevalence threshold. Epidemiological justification should include prevalence and incidence data, geographic distribution and diagnostic rates.
- There’s no satisfactory method of diagnosis, prevention or treatment authorised in the jurisdiction, or the new therapy offers a clinically significant benefit over existing options (improved efficacy, safety or patient quality of life).
- There is sound scientific rationale (including pre-clinical pharmacology, mechanism-of-action studies and early human data) plausibly predicting therapeutic potential.
Both comprehensive natural history and registry studies support prevalence estimates and unmet need arguments.
FDA orphan drug designation vs EMA orphan drug designation
Despite the FDA and the EMA offering similar incentives, their processes and requirements for ODD differ significantly. Understanding these differences is crucial for biotech or pharmaceutical companies navigating the global landscape of orphan drug development.
For example, the US relies on absolute patient numbers, whereas the EU uses prevalence rates, impacting diseases with low overall numbers but varying incidence by region.
Below is an overview of how orphan drug designation criteria differ between the FDA and EMA.
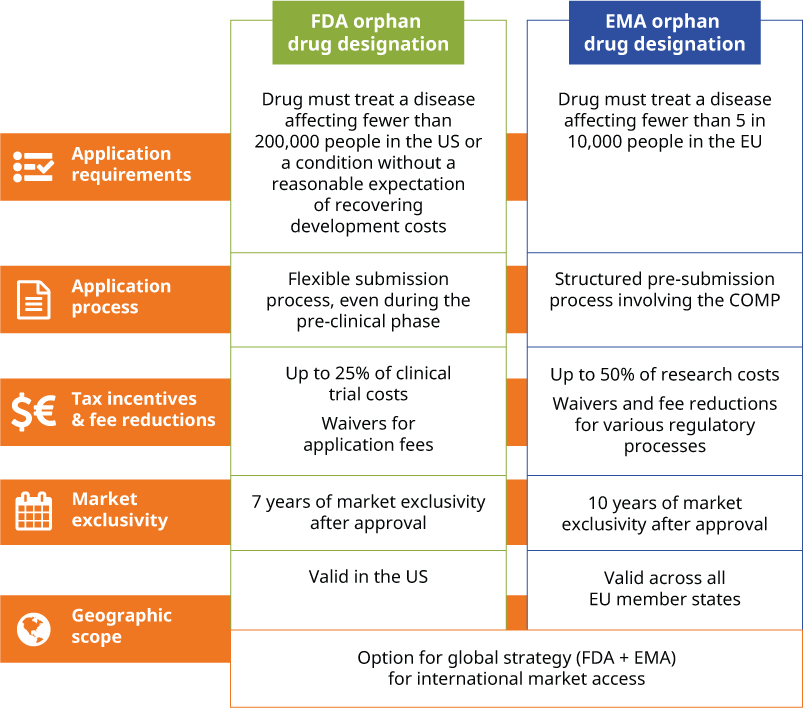
The choice between applying for FDA or EMA orphan drug designation largely depends on your target market, the geographic scope of the product and your drug development stage. If you have a global strategy, you may choose to pursue an ODD with both agencies, allowing you to leverage the benefits of exclusivity and incentives in the US and Europe simultaneously.
2. What is the orphan drug designation application process?
Securing orphan drug designation requires meticulous planning and rigorous documentation. It’s important to ensure parallel submissions do not conflict. As such, you should map timelines to make sure your global strategy remains cohesive.
Here’s a step-by-step look at the ODD application process and what you need to action at each step:
3. What are the benefits of orphan drug designation?
Now you know more about the criteria for obtaining ODD, let’s discuss the key advantages.
Why is it worth seeking this special status? Mainly because securing orphan drug designation at an early stage dramatically reshapes the commercial and regulatory landscape for you if you’re targeting a rare disease with your new drug.
Here’s how an ODD can aid fundraising and enable continued drug development:
Market exclusivity
FDA orphan drug designation confers seven years of market exclusivity in the US post-approval. During this period, no other sponsor may receive approval for the same indication, barring a major clinical advantage demonstration. EMA orphan drug designation grants 10 years of similar exclusivity across EU member states, extendable by two additional years for compliance with paediatric investigation plans (PIPs).
This window of exclusivity protects your orphan drug from direct competitors and enhances investor confidence, which can help with pricing leverage during negotiations with payers.
Regulatory fee incentives
FDA orphan drug designation sponsors receive full waiver of New Drug Application (NDA) and Biologics License Application (BLA) user fees — savings that can range into millions of dollars. EMA orphan drug designation offers significant reductions on marketing authorisation application fees and protocol assistance costs through the EU’s fee reduction scheme.
Reducing upfront regulatory expenses reallocates budget to your critical clinical trials and manufacturing scale-up.
Grant funding and tax credits
In the US, the Orphan Products Grants Program awards up to $1 million per award for Phase I/II clinical studies. You can also claim a 25% tax credit for qualifying clinical research expenditures under Section 45C of the Internal Revenue Code.
In Europe, national bodies and the EU’s Horizon 2020 framework provide research grants and innovation funding dedicated to rare diseases, offsetting early-stage R&D costs.
Scientific advice and protocol assistance
The FDA orphan drug designation triggers eligibility for the Office of Orphan Products Development’s advisory meetings, providing tailored feedback on study design and endpoints before you start pivotal trials.
The EMA’s Protocol Assistance offers a formal mechanism to refine your trial protocols, validating surrogate endpoints or adaptive designs suited to small populations.
Engaging regulators early minimises the risk of later-stage protocol amendments and rejection.
Enhanced visibility and stakeholder engagement
Orphan drug status increases your project’s visibility, attracting interest from patient advocacy groups, KOLs and specialised clinical research organisations (CROs). Collaborations with patient organisations can help you accelerate recruitment for clinical trials, while patient-reported outcomes enrich your data package with real-world evidence.
These strategic alliances support both scientific rationale and marketing narratives, enhancing reimbursement discussions upon approval.
4. What are the common challenges associated with orphan drug designation?
Although there are many advantages of obtaining ODD, the process can present several hurdles that can delay or complicate the application.
Challenge 1: limited patient populations
One of the most fundamental challenges in pursuing orphan drug designation lies in the inherently limited patient populations. Rare diseases by definition affect small, often widely dispersed cohorts, making it difficult to establish robust prevalence estimates. This scarcity also slows the recruitment of participants into clinical studies, prolonging timelines and increasing costs.
Challenge 2: epidemiological uncertainty
Closely linked is the issue of epidemiological uncertainty. Many rare diseases suffer from underdiagnosis or misdiagnosis, and registries that track these conditions are still evolving or fragmented. As a result, data on disease prevalence and incidence can be inconsistent, complicating efforts to meet the stringent orphan drug designation criteria required by regulators.
Challenge 3: diagnostic heterogeneity
Adding to these obstacles is diagnostic heterogeneity. Without universally accepted diagnostic standards, clinicians across different regions or even within the same country may use varied criteria to identify patients. This inconsistency can be challenging if you’re trying to define a clear, uniform target population in your application.
Challenge 4: evolving regulatory guidance
The regulatory landscape itself also presents moving targets. Rapid advancements in science — especially the surge of gene and cell therapies — often outpace existing frameworks and guidelines. If you’re developing a truly novel approach, you may find that agency expectations are still evolving, leading to uncertainties around acceptable endpoints, comparators or long-term safety data.
Challenge 5: data gaps and resource constraints
Data gaps and resource constraints add another layer of complexity. Many rare diseases lack comprehensive natural history studies or validated biomarkers, forcing you to invest heavily in foundational research even before starting your pivotal trials. At the same time, the high cost of early-phase development can be a strain on your budget.
Challenge 6: global variability
Navigating the global regulatory environment can also be demanding. Differences between agencies in what constitutes sufficient dossier content, how fees are structured or how quickly reviews are conducted can make simultaneous submissions more resource-intensive. It’s essential to coordinate carefully to ensure you meet the diverse requirements across jurisdictions, adding both logistical and financial burdens to the drug development process.
Challenge 7: aligning with patient advocacy groups
Despite evolving engagement frameworks, coming to a mutually agreeable conclusion between the patient group, the sponsor and regulatory authorities is another, often overlooked, challenge.
An example of this challenge occurred during an ODD application for a rare developmental and epileptic encephalopathy (DEE) condition: cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD).
5. How to get an orphan drug designation
The aforementioned challenges are not uncommon when developing new treatments for rare diseases and highlight the complexities of the ODD application process.
Moving from theory to practice, here are six practical steps to optimise your path to orphan drug designation, including
1. Early and continuous patient organisation engagement
- Identify and collaborate with key advocacy groups to co-develop natural history studies, refine patient-centric endpoints and mobilise registries for clinical study recruitment.
- Incorporate patient representatives in protocol development committees to ensure trial feasibility and alignment with unmet needs.
2. Transparent trial design and endpoint selection
- Share early protocol outlines with the FDA/EMA and patient stakeholders. Early feedback mitigates risks of endpoint rejection and accelerates ethics committee approvals.
- Utilise innovative designs suited for small populations — adaptive dose-escalation, basket trials across genetic subtypes — to conserve resources while increasing data yield.
3. Articulate unmet needs with real-world evidence
- Develop and validate PRO instruments that capture quality of life and functional improvements meaningful to patients and payers.
- Curate longitudinal data to contrast disease progression with intervention effects, strengthening benefit-risk narratives.
4. Make use of incentive programmes and grants
- Apply for multiple grant cycles to fund Phase I/II clinical studies, aligning scientific milestones with grant deliverables.
- Map and apply to EU funding streams that complement EMA orphan incentives, coordinating application timelines to avoid overlap.
5. Harmonise US and EU submissions
- Create core sections — mechanism of action, natural history, non-clinical data — common to both FDA and EMA packages, then append jurisdiction-specific modules.
- Synchronise pre-submission meetings, ensuring consistent messaging and data presentation to both agencies.
6. Robust data packaging and continuous update
- Use a centralised document control system to track epidemiological updates, new clinical trial results and diagnostic criteria changes.
- Where permitted, submit incremental data packages to agencies, demonstrating ongoing research momentum and data integrity.
By integrating these steps, you can anticipate regulatory expectations, mitigate common pitfalls and obtain orphan drug designation with greater confidence and speed.
You can also significantly improve your chances of securing ODD and gaining market authorisation approval with TMC’s pharma services. Our expert team helps you obtain the necessary scientific evidence to support your drug development and guides you through the application process.
We can also act as your marketing authorisation holder (MAH), if you don’t have a presence in Europe, to ensure compliance with all local regulations. As an established EU/EEA-based MAH with SME status, can be your EU/EEA representative — simplifying new drug approvals and helping you secure substantial discounts on EMA fees.
Contact TMC Consulting today at connect@tmcpharma.com to find out how our pharma consultancy services can support your application and help you gain ODD status with the FDA or EMA.
