Speed is rarely achieved by pushing one function harder.
It comes from creating cohesion between functions that rely on each other, so decisions made in one workstream don’t create rework or delays in another. That’s why pharma consulting is increasingly shifting from single-discipline support to joined-up, cross-functional delivery.
For emerging and mid-sized sponsors, the pressure points are familiar: lean internal teams, ambitious timelines and complex external requirements — especially during early-phase clinical development, when strategy and evidence generation are still evolving.
When regulatory, safety, scientific and quality activities run in parallel but not in alignment, it becomes harder to hit milestones on time, particularly around evidence readiness and documentation.
An integrated approach brings the critical disciplines together early, helping you anticipate downstream requirements, align decisions across functions and keep momentum through planning, execution and delivery.
Why integration matters more than ever
Drug development is designed to be iterative — but avoidable iteration (rework caused by misalignment) is where timelines slip. Common causes include:
- Different teams working from varying versions of the truth (data, assumptions, positioning or document sets).
- Decisions made for speed in one area create delays elsewhere (e.g. a rushed strategy that triggers multiple authority questions).
- Late discovery of compliance gaps that require corrective action, retraining or document remediation.
- Over-reliance on sequential handoffs between providers rather than shared ownership of milestones.
Integrated pharma services reduce these risks by creating a single coordinated plan, with shared priorities and clear governance across workstreams. Often this is delivered through a functional service provider (either a traditional model or the increasingly popular flex/hybrid approach), where an embedded partner provides consistent resourcing and oversight across multiple functions, aligned to programme milestones rather than isolated tasks.
The result is more support and better alignment — both of which accelerate delivery.
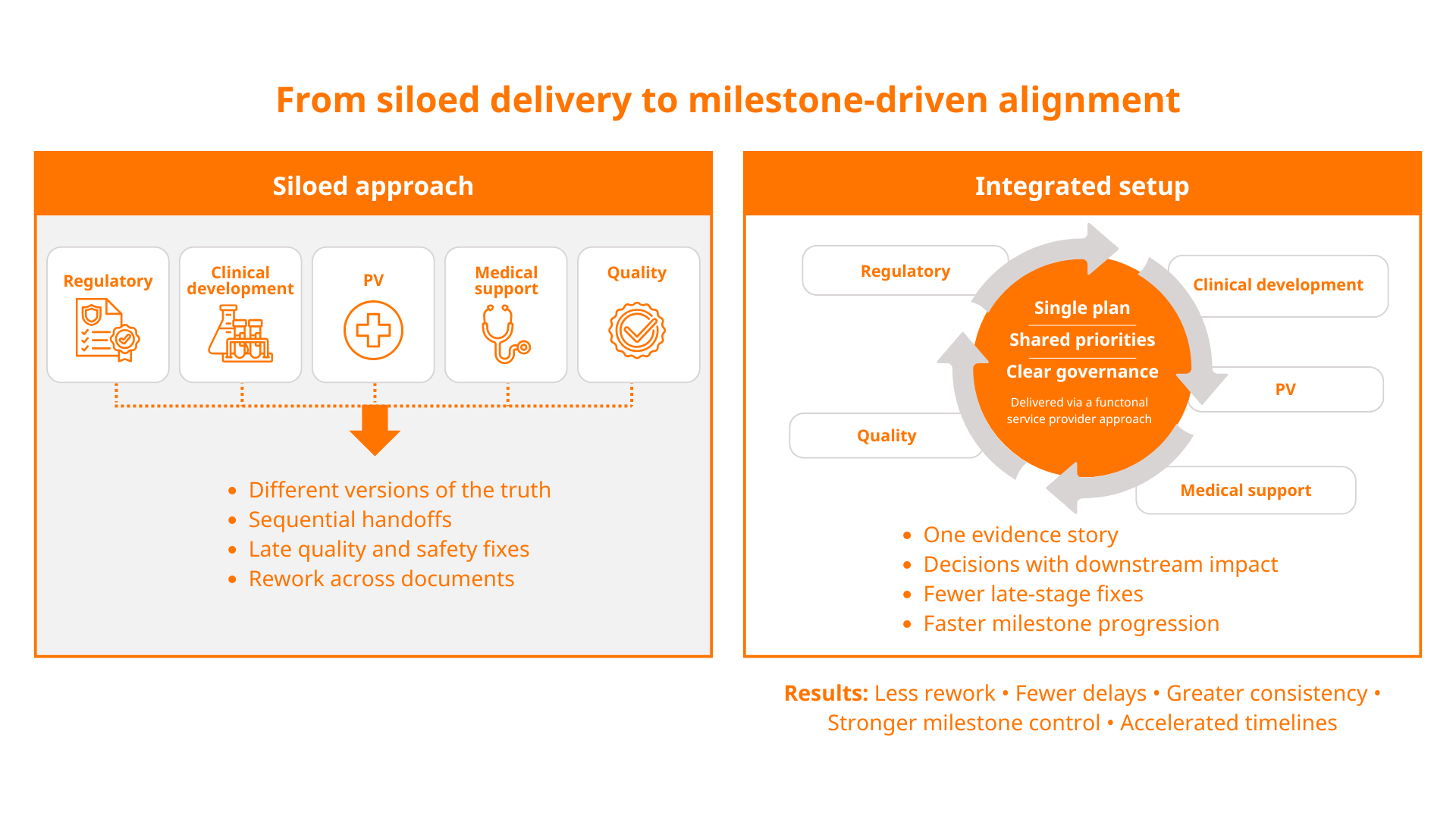
Where sponsors typically lose time and lack resources
Most delays in drug development don’t come from a single issue. They come from teams working in silos and small disconnects between functions that depend on each other, including regulatory approvals, clinical development, pharmacovigilance (PV), medical support and quality assurance.
These disconnects tend to show up at the worst possible moments: when timelines compress, teams are preparing regulatory submissions or programmes are transitioning from early-phase clinical development towards marketing authorisation and, ultimately, drug commercialisation.
Below are four common challenge areas where sponsors typically lose time or lack resources — and how a joined-up pharma consulting approach can help.
1. Regulatory readiness: keeping the strategy and evidence package coherent
Regulatory work rarely fails because a single document is missing. More often, programmes lose time because the strategy, evidence and messaging are developed in parallel, but not in alignment. When the narrative is not consistent across the submission package, it creates avoidable questions, rework and delays.
The challenges sponsors face
Regulatory strategy can become fragmented when inputs arrive late or from disconnected sources. Sponsors often encounter:
- Inconsistent positioning across documents (e.g. differing descriptions of benefit-risk rationale).
- Gaps in evidence linkage between clinical plans, safety content and quality expectations.
- Late changes, triggered by internal review cycles, that don’t reflect cross-functional impacts.
- Compressed timelines for regulatory submissions, which increase the risk of avoidable authority questions.
In short, regulatory submissions are rarely delayed because of one missing section — they’re delayed because the overall narrative and evidence package are not fully coherent.
How integrated support accelerates progress
An integrated operating model helps build regulatory readiness earlier by ensuring that regulatory decisions are informed by safety, medical input and quality considerations from the start. Practical benefits include:
- Earlier alignment on the regulatory narrative: a shared view of claims, evidence and rationale reduces late-stage rewrites and keeps documents consistent.
- Fewer disconnects across deliverables: safety content, quality considerations and scientific messaging are planned together to support the same strategy.
- Cleaner execution for regulatory submissions: coordinated planning reduces last-minute document churn and improves responsiveness when questions arise.
- Better continuity through marketing authorisation: integrated pharma services support the transition from clinical trial application submission right through to post-approval obligations without rebuilding context.
By reducing contradiction, duplication and rework, pharma consulting becomes a speed enabler.
2. Clinical development delivery: keeping early plans, evidence generation and execution aligned
Clinical development is where strategy meets reality. Even with a strong target profile and regulatory intent, programmes lose time when clinical plans are built in isolation from operational feasibility, safety requirements and the evidence narrative needed for regulatory approval.
The result is often avoidable amendments, inconsistent documentation and execution challenges that ripple into timelines and cost.
The challenges sponsors face
Sponsors commonly encounter pressure points that slow progress and add risk, including:
- Protocol concepts that are not fully stress-teste during the feasibility process, leading to slow start-up, recruitment issues or early amendments.
- Late incorporation of safety and quality requirements into study protocols, which creates documentation churn and operational disruption.
- Fragmented vendor oversight and unclear decision-making, especially when internal teams are lean and timelines are compressed.
- Inconsistent clinical documentation across protocol and operational materials, increasing review cycles and rework.
- Misalignment between clinical objectives, endpoint strategy and the evidence package required for marketing authorisation.
These issues are most acute in early-phase clinical development, where small design changes can have outsized downstream impact.
How integrated support accelerates progress
Integrated delivery helps sponsors protect timelines by aligning clinical planning and execution with regulatory, safety, medical writing and quality expectations from the outset. Key benefits include:
- Earlier feasibility and operational realism: protocol concepts are assessed against recruitment, site burden, country strategy and practical delivery constraints before they harden into avoidable amendments.
- Stronger protocol and evidence coherence: clinical objectives, endpoints and assessments are aligned to the wider programme narrative, supporting cleaner regulatory submissions and fewer late rewrites.
- Faster start-up through coordinated readiness: country and site activation activities are supported by aligned documentation, governance and decision pathways, reducing stop-start execution.
- More reliable vendor and stakeholder coordination: integrated oversight reduces handoff delays and creates clearer accountability across pharma services companies and other specialist providers.
- Better continuity into later stages and drug commercialisation: as data mature, the clinical story remains consistent across documents and workstreams, supporting downstream scientific communication, market planning and launch readiness.
When clinical development is integrated rather than run as a standalone workstream, sponsors spend less time correcting course — and more time hitting milestones with confidence.
3. Safety readiness: preventing pharmacovigilance from becoming a late-stage bottleneck
Safety obligations are time-sensitive, highly regulated and tightly linked to both regulatory and quality expectations. If PV planning starts late, sponsors can find themselves building systems under pressure — which is when errors, inconsistency and delays are most likely.
The challenges sponsors face
Safety obligations can quickly become a bottleneck when planning starts too late. Sponsors commonly struggle with:
- Safety systems and processes being set up reactively rather than proactively.
- Unclear roles and handoffs across vendors, partners and internal teams.
- Incomplete alignment between safety outputs and what is needed for regulatory submissions.
- Escalation pathways and governance that aren’t tested until an issue occurs.
When pharmacovigilance is treated as a late add-on, it can drive delays at exactly the moments when timelines are most fragile.
How integrated support accelerates progress
Integrated delivery supports speed by building safety readiness in parallel with the regulatory plan and clinical activities. This typically enables:
- Earlier definition of safety requirements: processes, reporting expectations and documentation are established before pressure peaks.
- Consistency across safety and regulatory outputs: PV inputs are aligned with the regulatory narrative, reducing rework and the risk of misaligned messages.
- Faster response cycles: shared governance and a single integrated plan improve turnaround when issues arise or updates are required.
- More reliable inspection readiness: safety systems and documentation are maintained in a steady state rather than rushed into place.
When pharmacovigilance is integrated with regulatory and quality from the outset, sponsors spend less time untangling dependencies — and more time moving forward.
4. Evidence and scientific alignment: avoiding late rework that slows development and submission readiness
Clear, consistent scientific communication is not a ‘nice to have’ at the end of a programme. The way you define the scientific rationale, endpoints and clinical relevance early on shapes feasibility decisions, evidence generation and the credibility of your regulatory narrative.
When scientific input and documentation are developed in parallel but not aligned, sponsors can lose time through avoidable rewrites, inconsistencies across deliverables and last-minute repositioning.
The challenges sponsors face
Sponsors often recognise the importance of scientific alignment — but underestimate how early it needs to start. Common issues include:
- Scientific rationale and evidence framing that drift from the regulatory strategy as plans evolve.
- Inconsistent interpretation of endpoints, comparators, target population or clinical relevance across teams and vendors.
- Limited capacity for high-quality medical writing, leading to documents that are technically correct but not strategically coherent.
- Early development decisions made without sufficient medical input, increasing the risk of downstream protocol amendments or feasibility failures.
- Scientific advice preparation that is rushed, with unresolved cross-functional questions surfacing too late to address cleanly.
Without structured coordination, the scientific story can become something teams retrofit late, rather than a thread that runs consistently from early engagement through submission.
How integrated support accelerates progress
Integrated delivery brings scientific input and documentation discipline forward, so you build a coherent evidence story alongside development. Key benefits include:
- Stronger early engagement in drug development: medical input is built into planning from the outset, helping align target product thinking, endpoints and evidence needs with what regulators are likely to expect.
- Higher-quality, more consistent medical writing: core documents are developed against a single agreed narrative, reducing contradictions across protocols, synopses, briefing packs and submission components.
- Medical plans creation that supports decision-making: structured medical planning clarifies what evidence is needed when, and why — supporting prioritisation and reducing late-stage changes.
- More robust feasibility and protocol development: medical support strengthens feasibility assumptions and site/patient considerations, helping reduce avoidable amendments and operational disruption.
- Better scientific advice preparation and alignment to regulatory strategy: integrated input ensures briefing materials, questions and justification are consistent with the wider programme strategy and evidence package, improving efficiency before and after meetings with authorities.
When medical input, writing and regulatory strategy are integrated rather than sequential, sponsors spend less time resolving inconsistencies — and more time progressing the programme with confidence.
5. Quality and compliance readiness: preventing compliance issues from becoming schedule issues
Quality underpins credibility with regulators, partners and internal governance. Even if you have quality processes in place, the risk lies in how consistently they are applied across teams, vendors and fast-moving timelines.
The challenges sponsors face
Quality is often assumed to be covered — until an inspection, audit, partner requirement or a key submission deadline exposes gaps and forces urgent remediation. Sponsors frequently encounter:
- Quality systems being built around immediate needs rather than sustainable compliance.
- Documentation and training lagging behind operational reality.
- Inconsistent traceability between processes, data handling and controlled documents.
- Disconnected expectations across clinical operations, safety and external partners.
Quality gaps rarely stay contained and can slow timelines dramatically — with the impact often landing at critical points. They typically create knock-on delays through document rework, slowed approvals and time-consuming reconciliation between vendors, systems and controlled records.
How integrated support accelerates progress
Integrating quality with regulatory, safety and scientific workstreams creates a more stable operational foundation and reduces late corrective actions. In practice, this supports:
- Right-first-time documentation: controlled documents are created with the end-use in mind, reducing remediation and version churn.
- Aligned governance and decision-making: quality oversight supports consistent execution across vendors and functions.
- Inspection and audit readiness as a continuous state: rather than a last-minute project, readiness is embedded into routine operations.
- Better alignment with PV processes: quality oversight supports compliant safety processes and documentation across the programme, strengthening reliability when timelines tighten.
This is one of the most direct ways integrated pharma services protect speed: by preventing compliance issues from becoming schedule issues.
Building speed and alignment through integrated pharma services
Hitting key milestones faster depends on reducing the avoidable complexity that emerges between regulatory, pharmacovigilance, medical and quality — not just optimising each function in isolation.
Done well, integrated pharma services bring earlier clarity, reduce rework, and strengthen readiness across the lifecycle, helping sponsors move through pivotal moments with fewer delays and stronger consistency.
This is where pharma consulting delivers its biggest impact: connecting strategy and execution so the evidence package, governance and decision-making stay aligned as timelines compress and requirements increase.
TMC Consulting provides expert-led strategic and operational pharma consulting spanning regulatory support, PV, medical services and quality management. Our specialists integrate into your team to provide scalable support that helps you reach critical milestones compliantly, quickly and cost-effectively, while keeping regulatory direction aligned with downstream market entry needs.
If you’re exploring how integrated cross-functional support could help you protect your next milestone, speak to our team today at connect@tmcpharma.com.
