Submitting a dossier to the EMA or MHRA is a narrative that proves your product’s safety, efficacy and quality.
Clear, compliant and strategic medical writing can mean the difference between delays and a smooth drug development pathway. However, small to mid-sized biotech and pharma companies with limited infrastructure may lack the resources and expertise for effective writing and regulatory submissions.
This blog explains how good writing supports clinical study reports (CSRs), investigator brochures (IBs) and common technical document (CTD) modules — and how it can aid regulatory submissions to the EMA or MHRA.
Why focused medical writing matters for regulatory submission outcomes
Regulatory authorities like the EMA and MHRA expect dossiers that are logically structured, harmonised across modules and transparent about benefit–risk. High-quality writing turns complex datasets and clinical jargon into submission-ready documents that reviewers can navigate quickly, reducing clarification requests and accelerating timelines.
While medical writing supports drug development from early stages, its regulatory impact is most visible across Phase I–III: IBs guide first-in-human safety, Phase II/III CSRs provide efficacy and safety evidence for registration, and Module 2 narratives synthesise outcomes for the regulatory submission process. Post-marketing activities (Phase IV) also use medical writing for safety updates and study reports, but the core CTD/marketing authorisation application work lives in the pivotal Phase I–III timeline.
Where medical writing adds the most value
For EMA/MHRA regulatory submissions, medical writing has three high-impact roles:
1. Clinical study reports (CSRs)
The ICH E3 guidance defines the expected structure and content of CSRs.
CSRs are the primary source of clinical evidence in CTD Module 5. Medical writers ensure CSRs follow ICH structure, present methods and results clearly, and integrate tables, figures and appendices so that reviewers can validate analyses without searching for missing pieces.
Standardised, readable reports are a requirement for any regulatory submission. A well-crafted CSR anticipates queries by clearly documenting protocol deviations, statistical methods and safety analyses — reducing back-and-forth with regulators.
2. Investigator brochures (IBs)
The IB translates preclinical and clinical data into a single, actionable risk–benefit picture for investigators and ethics committees.
Medical writing keeps the IB current through development (Phase I to Phase III), highlights safety signals and frames tolerability in clinically meaningful terms so that trial sites and regulators understand the rationale for your study design and risk mitigations.
3. Common technical document (CTD) modules and clinical overviews
Medical writers synthesise individual CSRs into Module 2 (Clinical Overview and Summaries) and ensure Module 5 is organised and cross-referenced. The CTD is a coordinated story: readable clinical summaries (Module 2.5, 2.7) must reflect the raw and tabulated evidence in Module 5.
Harmonising language and data across modules is where medical writing creates regulatory clarity and trust. The ICH M4 guidance explains the CTD organisation and expectations for Module 5.
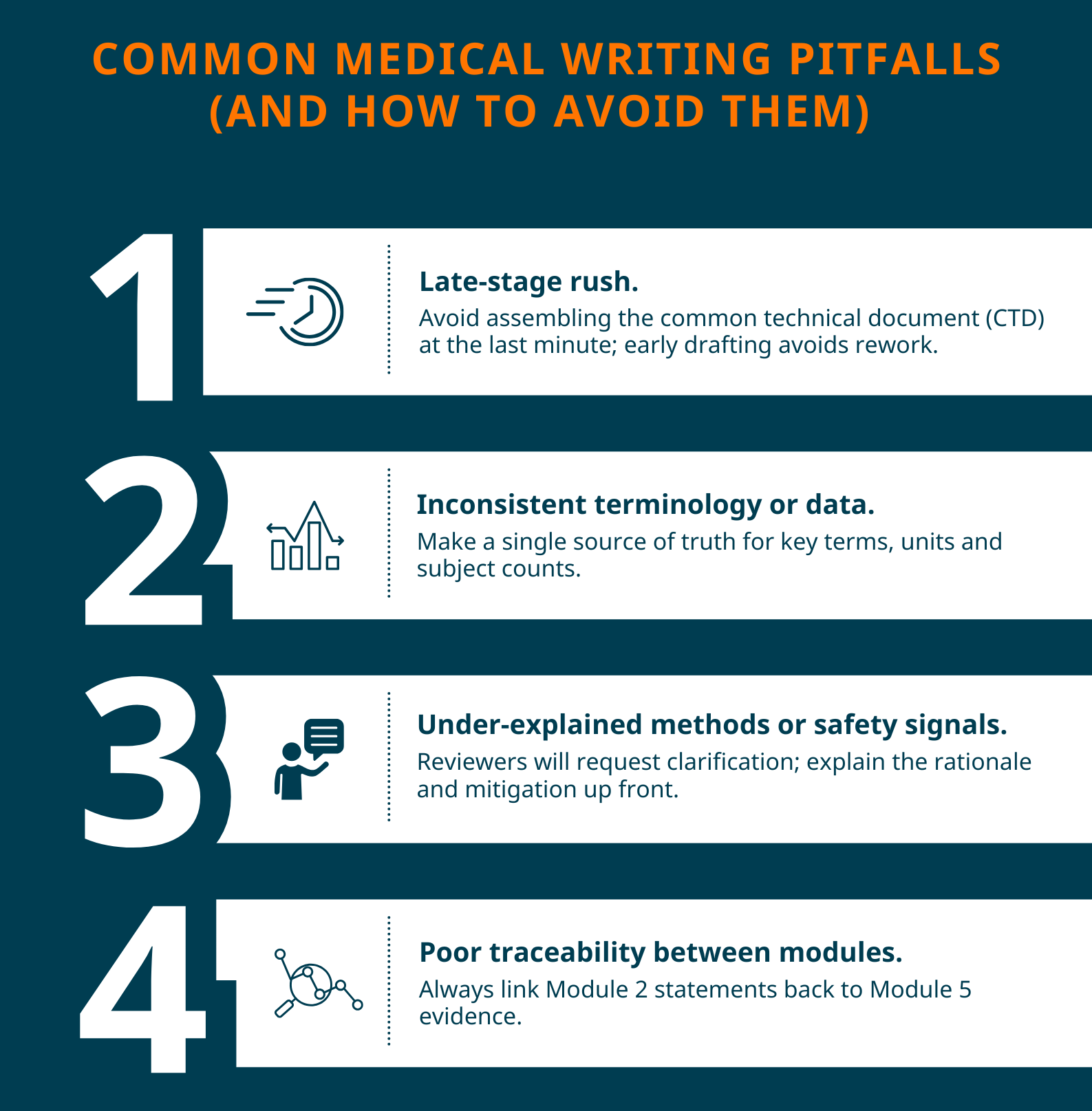
To increase chances of gaining approval during the regulatory submission process, you should:
- Start early and iterate. Draft IB and CSR templates during protocol design and update them as data accrues. Early alignment avoids rushed rewrites at submission time.
- Use standard headings and templates. Following ICH E3 and CTD conventions reduces review friction.
- Cross-reference relentlessly. Ensure Module 2 claims are traceable to Module 5 tables/appendices. Auditors and regulators value traceable evidence.
- Flag limitations and mitigation strategies. Transparent discussion of limitations demonstrates robust scientific thinking and risk management.
- Quality control for consistency. Run a targeted editorial and clinical check for data consistency, units, subject counts and adverse event coding before submission.
Integrating medical writing into your outsourced model
Small to mid-sized biotech and pharma companies typically complement in-house expertise with external partners. When choosing partners to assist with the regulatory submission process, including medical writing, look for providers that offer end-to-end pharma services and a consultative approach.
- Teams that combine clinical, statistical and regulatory experience avoid siloed drafts.
- Providers that offer pharma consulting alongside writing can help shape submission strategy (e.g. how to present an adaptive design or interim analysis).
- Integration with medical affairs ensures public-facing materials and scientific communications are aligned with the submission narrative.
- If you need a full-service option, seek vendors offering integrated medical services (medical affairs and writing plus regulatory strategy) to simplify handoffs and strengthen message consistency.
These integrated approaches make the submission narrative coherent and defendable during review, inspection or advisory committee interactions.
A partner that combines pharma services, pharma consulting and integrated medical services reduces risk. They bring regulatory experience tailored to EMA/MHRA expectations and the editorial discipline to produce submission-ready CSRs, IBs and CTD modules — freeing your internal team to focus on clinical execution and sponsor responsibilities.
TMC Consulting provides expert-led strategic and operational pharma consulting services — spanning regulatory support, clinical development, pharmacovigilance, medical services and quality management. Our experienced specialist team partners flexibly with you to help you reach critical drug development milestones like regulatory submissions compliantly, quickly and cost-effectively.
Contact our team today at connect@tmcpharma.com to learn more about our pharma services and how we can help you accelerate innovative treatment access for patients who need them most.
