Between 2020 and 2023, 173 innovative medicines received marketing authorisation across 36 European countries. However, nearly half (48%) of these medicines were not available to patients in 2024.
Only 29% of products were fully available on public reimbursement lists, and 17% of products were available with restrictions.
Bringing a medicine from concept to clinic and then to market is a long, complex journey. Yet, many first-time sponsors believe regulatory approval is the ultimate objective. In reality, it’s just one milestone in a far bigger, interconnected process.
Without early alignment on your commercial goals and pharma market access strategy, even the most promising therapies can stall before reaching patients.
In this blog, we’ll break down the five critical stages of the drug commercialisation process, expose common pitfalls that could derail your drug development timelines and share practical insights to help you move from breakthrough science to real-world impact — faster and with fewer surprises.
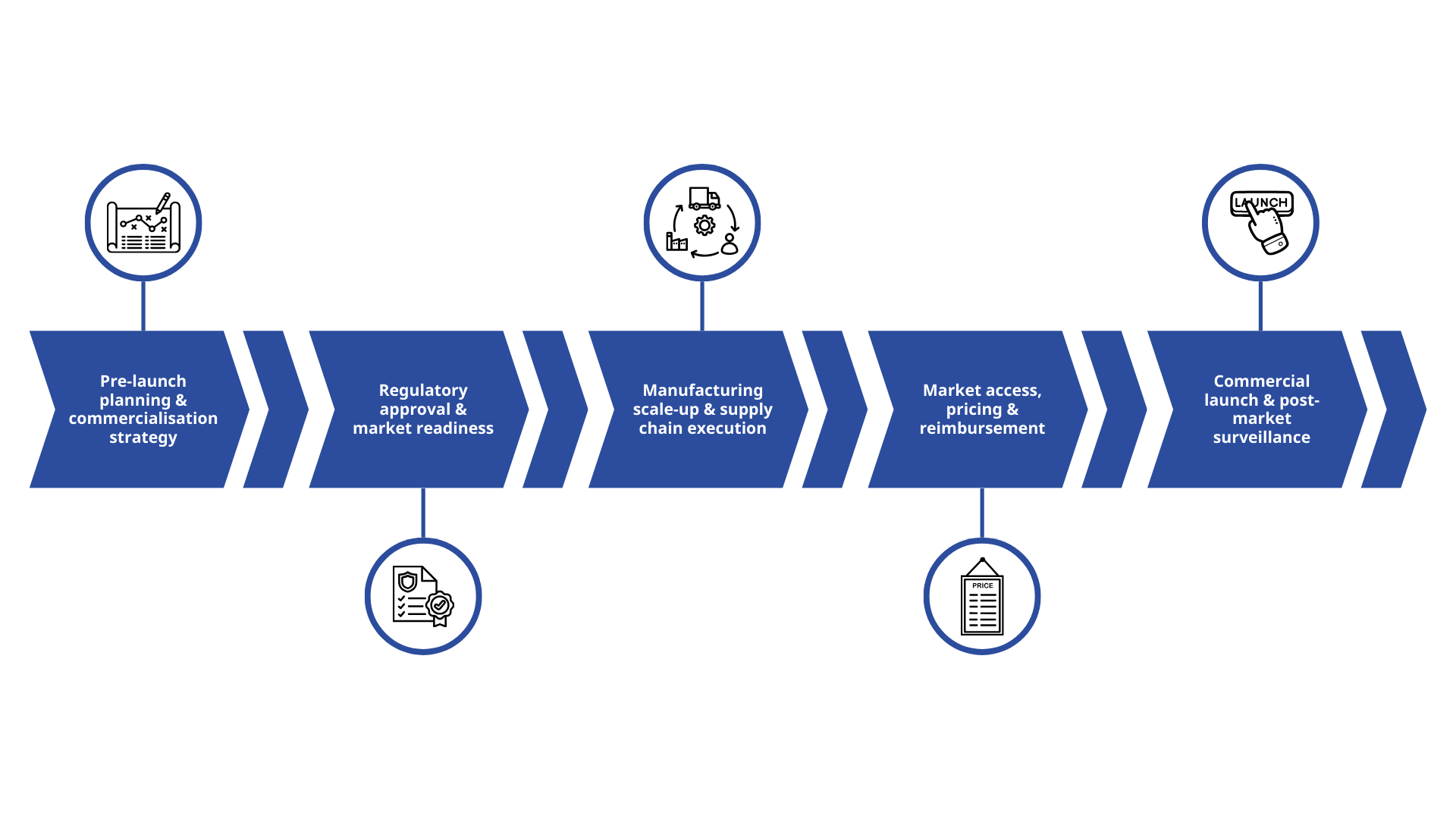
1. Pre-launch planning and drug commercialisation strategy
Pre-launch planning lays the foundation for every decision that follows in the drug commercialisation process. At this stage, you should define what success looks like — clinically, commercially, and operationally — while considering how early choices will impact your pharma market access strategy later on.
This phase includes market and competitive landscape assessment; target product profile (TPP) development; stakeholder mapping (patients, clinicians, payers, regulators); early health economics and outcomes research (HEOR) planning; and Joint Clinical Assessment (JCA) preparation. These activities are essential for shaping a strong value proposition that supports future marketing authorisation submissions and aligns with long-term commercial goals.
Limited understanding of payer expectations, endpoints that fail to translate into real-world value and underestimating commercial complexity for challenging indications are common challenges here. Solutions include aligning clinical and commercial teams early, pressure-testing the value proposition with external experts and using real-world insights to refine target populations.
When executed effectively, this stage delivers stronger differentiation in crowded drug development pipelines, clearer messaging for regulators and payers, and reduced risk of costly late-stage redesigns — all of which set the stage for successful commercialisation and compliance with marketing authorisation holder requirements later in the journey.
2. Regulatory approval and market readiness
Achieving marketing authorisation is a pivotal milestone that shapes how a product can be positioned, what claims can be made and what post-approval commitments will follow. This stage involves preparing and submitting marketing authorisation applications, negotiating labels and indications, planning for risk management and pharmacovigilance, and aligning on post-marketing study requirements.
For first-time sponsors, navigating differing regulatory expectations across regions, managing complex requirements for advanced therapies, and ensuring chemistry, manufacturing and controls (CMC) readiness can be daunting. These challenges can be mitigated through early engagement with health authorities, proactive planning for post-approval obligations and leveraging integrated regulatory and operational expertise.
When done well, this stage delivers faster review timelines, fewer post-approval surprises and a clearer path to launch — critical for accelerating drug commercialisation.
3. Manufacturing scale-up and supply chain execution
Transitioning from clinical supply to full-scale commercial manufacturing is one of the most operationally demanding phases of the drug commercialisation process, especially for biologics and advanced therapies. Sponsors must ensure manufacturing readiness, validate processes, transfer technology effectively and establish robust supply chain and logistics plans, all while maintaining quality systems and audit preparedness.
Common pain points include limited internal manufacturing experience, capacity constraints and dependency on external vendors. These risks can be reduced by aligning manufacturing strategy with demand forecasts early, selecting suppliers based on risk profiles and implementing strong quality and governance frameworks.
The payoff is significant, including reliable product availability, reduced compliance risk and improved cost control.
4: Market access, pricing and pharma reimbursement
Regulatory approval, and even JCA submission, doesn’t guarantee patient access. A strong pharma market access strategy determines whether patients can receive treatment and whether the product achieves commercial viability. This stage requires developing country-specific pricing and pharma reimbursement strategies, preparing health technology assessment (HTA) submissions, generating evidence to demonstrate value and engaging with payers and health systems.
Sponsors often struggle to prove value, navigate inconsistent pharma reimbursement pathways across countries and address budget impact concerns. These challenges can be overcome by building evidence strategies early, integrating these strategies into pivotal study design with real-world evidence planning and access approaches tailored by geography.
Effective execution accelerates patient access, strengthens pharma reimbursement outcomes and supports sustainable long-term revenue — key goals of any drug commercialisation process.
5: Commercial launch and post-market surveillance
Launching a product is just the start. For many first-time sponsors, the real challenge isn’t sales or awareness; it’s compliance.
Compliance obligations such as pharmacovigilance, risk management and post-market surveillance continue long after launch, and fragmented governance often leads to gaps in safety reporting and lifecycle management.
The marketing authorisation holder role is particularly critical post-approval to ensure your product consistently meets the stringent safety, efficacy and quality standards required by regulatory authorities.
However, acting as a marketing authorisation holder comes with complex regulatory obligations that most emerging biotech and pharma companies simply aren’t equipped to handle. Without the right infrastructure, you risk delays, compliance breaches and reputational damage.
If your infrastructure is limited, you should secure an experienced partner who can act as your marketing authorisation holder and manage obligations end-to-end, while integrating compliance planning into the overall launch strategy.
Handled well, this stage delivers far more than strong uptake and patient engagement — it ensures your product remains compliant, safe and competitive throughout its lifecycle.
Move through the drug commercialisation process quickly and confidently
If you’re a first-time sponsor, the drug commercialisation process doesn’t have to be daunting. With the right understanding of each stage (and early, integrated planning), you can reduce risk, accelerate timelines and maximise patient access.
TMC Commercial provides flexible, comprehensive UK and EU drug commercialisation services — from preparing for submission to post-authorisation — giving you the opportunity to build your own commercial presence without needing to out-license to a partner. Our drug commercialisation solutions also include the expertise to act as your marketing authorisation holder.
We help you make informed, long-term decisions that reduce the time, cost and other complexities associated with market entry, ensuring regulatory compliance to de-risk your commercial activities and allowing you to retain control of your asset without the operational burden.
TMC Commercial helps you launch and scale with speed and confidence across the EU and UK — within months, not years. Contact our team today at connect@tmcpharma.com to find out more.
